Vessi Medical
130% of funding target


Highlights
Highlights
Adaptation of proven and successful medical technology for the treatment of complex cancer
The innovative cryotherapy technology enables the treatment and elimination of cancer cells by freezing and thawing them. To date, it has been widely used in various cancers' treatments, but so far the anatomical complexity of the bladder did not allow this technology to treat superficial bladder cancer. Superficial bladder cancer counts for 75% of bladder cancers the fourth most common cancer among men, while presents in women as well with a lower incidence. In current treatments for this cancer, the cancerous cells are 'scraped’, yet most often, the treatment fails to eradicate them, and the disease recurs (50-80% chance of recurrence). Existing treatments are performed under general anesthesia, and repeated treatments significantly impair patients' quality of life.
For the first time, the cancer cell can be destroyed without harming healthy cells
Using state-of-the-art medical technology, VessiMedical's innovative device removes the barrier to freezing inside the bladder, allowing for the first time to treat this cancer through 'cold burn' (ablation) - rapidly freezing and thawing the cancerous cells, destroying them without affecting the patient's quality of life. In doing so, the company fundamentally changes the medical concept of how bladder cancer is being treated. The company is now following preclinical trials that have been crowned as an extraordinary success.
Huge market with demand for innovative technology
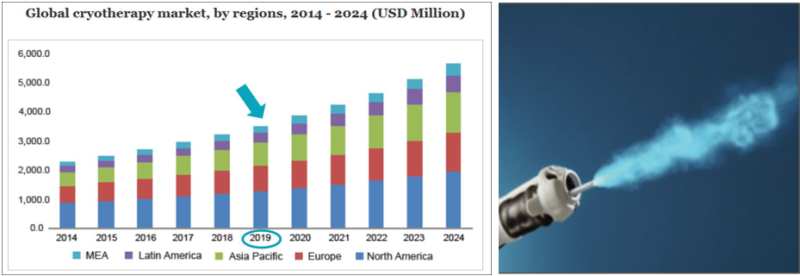
The innovative device developed by VessiMedical is relevant for a number of medical markets: (1) bladder cancer, the most expensive of all cancers for patient care, and the fifth most expensive in total medical expenses (about $ 3.7 billion a year). In this market, the cost of medical procedures relevant to the company is estimated at $ 1.2 billion a year, while about half of this market is in the U.S. VessiMedical's technology is expected to reduce tens of percent not only in direct medical care costs, but to significantly reduce general healthcare costs and expenses. (2) The technology enables additional non-cancerous bladder related treatments and Medical procedures that till now were not available - urinary tract refractory infections, overactive bladder, etc. These markets are valued at $ 15 billion a year.
.png)
The Product: Simple to Use and Prevents the Need for Surgery
VessiMedical's technology is simple to use because it is tailored to the existing doctor's expertise and training. In addition, unlike current treatments, it even allows the doctor to perform the procedure in an outpatient clinic - without surgery! The simplicity and ease of use of the medical device is expected to lead to rapid adoption of the product by professionals, specialist urologists around the world, and the lack of the need for in-hospital general anesthesia is likely to make this treatment popular among health systems, physicians and patients alike.
A Team Made Up of Experienced Professionals Who Identified A Great Need
Thanks to many years of experience and professional knowledge of the relevant medical field, the founders of VessiMedical realized that there is a great need for innovation in the treatment of superficial bladder cancer and embarked on a quest to find the right solution. The team's areas of expertise combine extensive technological know-how in medical engineering and a deep and multi-year familiarity with the world of cryotherapy and urology. VessiMedical's team has proven execution abilities, years of experience in connecting technological solutions with cancer care market, as well as with the business needs of those involved (doctors, healthcare systems, hospitals, and patients).
Trust by the largest medical tech incubator in Israel
VessiMedical has recently received an investment from the well-known medical innovation incubator "Trendlines Medical", Israel's largest seed investor in medical startups, in collaboration with the Israeli Innovation Authority (formerly the Office of Chief Scientist).
Pitch
Pitch
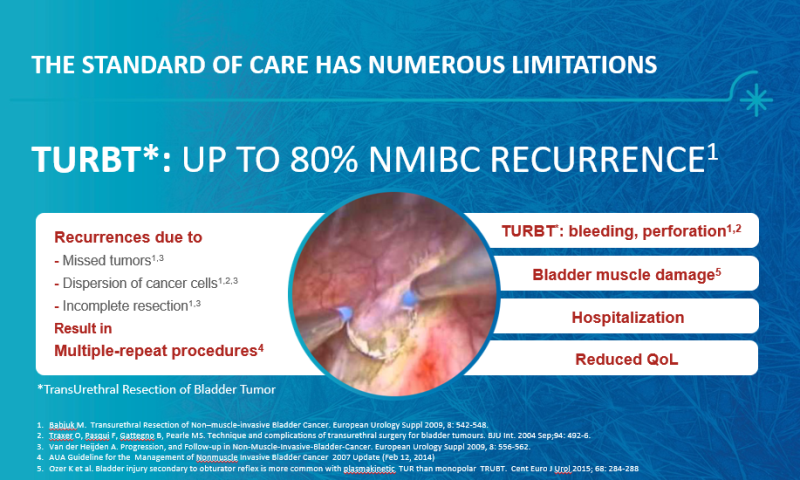
The Problem
Superficial bladder cancer (which doesn’t penetrate the bladder muscle) is most common in men over the age of 55. In Israel, bladder cancer rates are among the highest in the world and it ranks 11th, comparing to other countries, while among men it is even more prevalent and it rates 9th. This type of cancer is the fourth most common in men in the Western world (after prostate, lung and colon cancer) and accounts for between 5 to 10 percent of all men-related cancers in Europe and the United States. This cancer when being fairly diagnosed and treated, is rarely defined as a life-threatening situation, but the existing treatments have a significant impact on the quality of patients' lives; nowadays, the most common treatment for this cancer involves in-hospital surgery with general anesthesia. In this procedure, the doctor “scrapes” the cancer cells (following flushing inside the bladder with dedicated materials). This surgical procedure, while both expensive and invasive, is complicated to perform and may also harm the bladder, therefore, it is performed with the utmost care and delicacy though usually fails to prevent tumor recurrence (in about 50% to 80% of cases the cancer returns). Since most patients require repeat treatments, accumulating complications in patients’ urinary tract decreases quality of life - for example, Infection, bladder perforations, bleeding and other complications.
The Need
Cryotherapy is a cell-freezing technology used for various medical procedures and is used effectively in the treatment of various types of cancers (skin, kidney, lung and more). Using this technology, the cancer cells undergo rapid freezing and thawing, which eliminates them accurately and doesn’t leave any untreated areas. Due to the anatomical complexity of the bladder and the need to avoid significant injury to the organ itself during the procedure, alternative and innovative treatment methods have not been made available to date. The current technique injures and spreads cancer inside the bladder cavity and does not allow full identification of all cancerous cells and doesn’t support their complete removal, so the disease often recurs. Today's procedures are decades old, and using innovative alternatives such as Cryo spray to freeze cancer cells would have created a "fog" in front of the optical device currently used by the doctor, leading to an inability to visualize bladder’s interior during the procedure (which is critical). This is especially problematic when it comes to the bladder, which is an organ very sensitive to pressure and temperature.
the solution
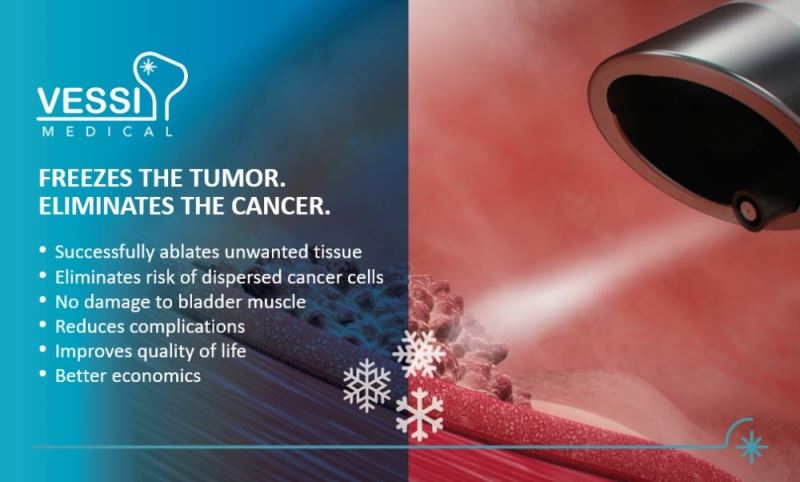
For the first time ever, the system developed by VessiMedical allows the treatment of superficial bladder cancer using cryotherapy technology as a replacement to surgery! The innovative treatment combines VessiMedical's unique ‘Cryo’ system with standard optics so that the doctor recognizes cancer and controls the accuracy of the treatment in real-time. The state-of-the-art technology balances the cooling temperature and the ideal degree of pressure, which is needed to target cancerous cells while fully protecting the bladder. The technology also solves the mist problem created when activating cryo-spray inside the bladder, enabling the treating physician to identify and "burn" (freeze and thaw) all cancerous targeted cells without bleeding or perforation, as demonstrated in preclinical trials.
Significant Benefits of VessiMedical’s Product:
• Successfully eliminates cancerous tissue.
• Eliminates the risk of spreading cancer cells.
• Prevents bladder damage and therefore improves patients’ quality of life and well-being.
• Reduces indirect complications from general anesthesia surgery.
• The disposable device, connected to VessiMedical’s dedicated console, is very suitable for the Western market that relies on disposables.
.png)
Team
Team
|
Prof. Gilad Amiel, is leading company’s clinical challenge and is part of its Scientific advisory - The Chairman of Rambam Health Care Campus’s Department of Urology in Haifa, Israel, and also an Adjunct Associate Professor at the Scott Department of Urology, Baylor College of Medicine in Houston, Texas where he worked for 11 years (2003-2014). Prof. Amiel previously served as the President of the American Association for Cancer Education (AACE).
|
|
Doron Birger company’s business advisor - Former President and CEO, Elron (NASDAQ-ELRN); former Chairman Given Imaging (NASDAQ-GIVN); chairman and board member of numerous medical device companies.
|
|
Moti Simchon, is leading company’s cryogenics and general technology – With 45 years of experience in pneumatics, fine mechanics, and cryogenics, with prior positions at the Israeli defense industry in the field of defense systems. With BSc, mechanical engineering, from the Technion Israel institute of Technology.
|
|
Prof. Yair Lotan, scientific advisor for the company - Professor of Urology, Chief of Urologic Oncology, and holder of Professorship in Urology at UT Southwestern Medical Center, Dallas, TX. He is also Medical Director of the Urology Clinic at Parkland Health and Hospital System, Dallas, TX. Prof. Lotan is a world leader in the field of bladder cancer.
|
|
Steve Rhodes, board member – B.A. in Near Eastern Languages and Civilizations at the Harvard University (1978) and an MBA in Finance and Marketing at the University of Chicago (1984). He is Co-Chairman/Co-CEO/Co-Founder of Trendlines Group Ltd, which was founded in 1993. As CEO of Trendlines, he founded Trendlines Medical in 2006 supporting new ventures whether in start-ups or established companies to develop innovative medical devices.
|
|
Dr. Nitza Kardish, board member – MSc in Plant Physiology (1986) at the Tel Aviv University, and PhD in Biochemistry and Physiology at the same university (1991). Post Doc in Plant molecular biology and genetic engineering at Weizmann Institute of Science (Israel). After being CEO and Business development manager of several companies such as TechnionSeed, Clal Life Science and UroGyn, she became CEO and VP of Trendlines Group.
|
|
Eran Hertz, board member – BA in Accounting and Economics at the Tel Aviv University (1998) and an MBA in Management of knowledge-based and technology-intensive firms at the Technion - Israel Institute of Technology (2012). He is a Certified Public Accountant. He collected a vast finance/accounting experience being, among the others, CFO at PlaySight Interactive Ltd., and CFO at Integrity Applications, Inc.
|







