ProArc Medical
112% of funding target
Highlights
Highlights
Solution for millions of men worldwide
ProArc’s technology addresses a crucial problem for millions of men worldwide. For many, medication is not sufficient, and they must undergo surgery to treat their Benign Prostatic Hyperplasia (BPH) to improve their quality of life. The ClearRing is a potential game-changer enabling a quick and simple procedure for restoring normal and healthy urinary function while eliminating and possible irreversible damage to sexual function that is common with treatment involving prostate tissue removal.
A Rapidly growing market with M&As at high valuations
Market demand for minimally invasive treatments of BPH is rapidly growing driven in large part by a growing population of men over the age of 50. This multi-billion-dollar market has become the target of large healthcare companies, resulting into several significant acquisitions.
Clinically validated technology, backed by broad IP protection
ProArc’s technology was validated in a first-in-man feasibility multi-center study conducted in Israel and abroad. That study demonstrated the safety profile and potential efficacy of this unique solution, specifically the improvement of urological function without sexual side effects along with quick patient recovery.
The clinical progress of ProArc is accompanied by a strong IP portfolio including already two granted patents in the U.S. and two granted patents in Europe.
Experienced healthcare investors along with IIA and EU SME Horizon 2020 grants
In 2019, ProArc Medical announced that it had been awarded a significant grant from the EU's Horizon 2020 program, in addition to prior funding by the Israel Innovation Authority. The two-year grant supports further R&D and clinical studies with the goal of commercialization of ProArc's ClearRing BPH solution.

Executive Management Team
ProArc is led by seasoned executives with track records of bringing ideas to advanced commercialization, M&A & IPOs. David Nitsan, CEO, Robert Bash, Chairman, and Eli Gendler, CFO have all participated in bringing med-tech startup companies to significant growth and value.
Pitch
Pitch
The Need
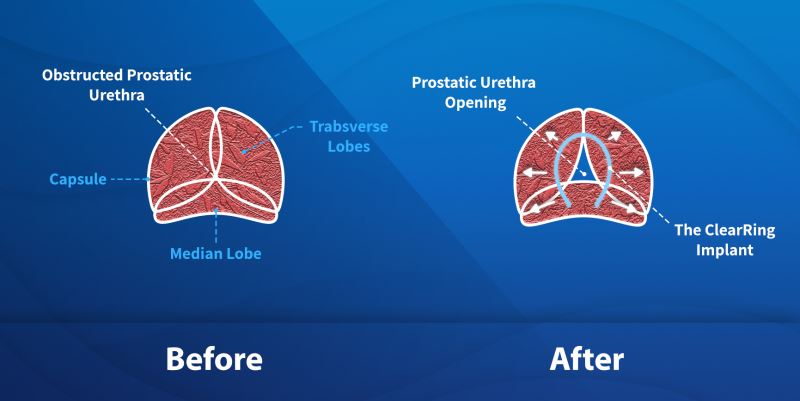
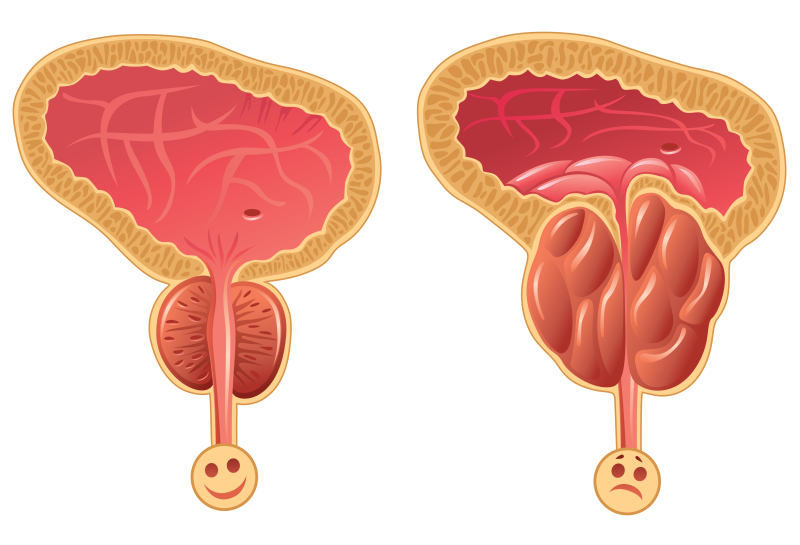
BPH is one of the most common urological diseases among men, affecting 40-50% of men in their 50’s and 80-90% of men in their 80’s, and its prevalence is growing due to the aging of the population. While BPH is a benign (non-cancerous) condition, it can cause loss of productivity and sleep, depression & sexual dysfunction. As a result, this aging population is driving increased healthcare expenditures and the search for cost-effective solutions for improving outcomes. In the field of BPH, patients are demanding enhanced quality of life that preserves sexual function which cannot be delivered by the ‘gold-standard’ of more invasive surgical procedures.
The Solution
ClearRing is positioned to be a game-changer in the treatment of BPH, enabling fast, minimally invasive procedures and recovery, improvement in urological function with preserved sexual function. With ClearRing, there is no need for ongoing BPH medications, or the removal of prostate tissue and the procedure is fully reversible. Minimally invasive BPH procedures are high-value and covered by public and private insurance.
Novelty of the innovation with respect to the state of the art
- Common BPH treatment options include medication, surgery such as an open prostatectomy, transurethral resection of the prostate (TURP), and minimally invasive therapies such as transurethral needle ablation (TUNA), laser therapy, radiofrequency ablation, urethral lift, water and vapor ablation, and permanent or temporal stents implants.
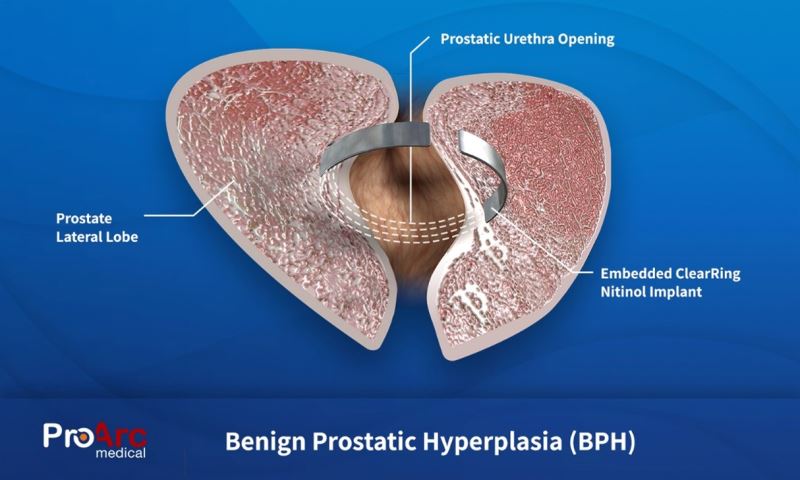
- The ClearRing system delivers a metallic device, that once implanted into the tissue of the prostate, reshapes the prostate to enable the healthy flow of urine through the urethra. Unlike other implants, the ClearRing does not come into contact with urine that can lead to encrustation or migration nor does it involve any traumatic removal or ablation of prostatic tissue.
- The implant is delivered via a fully automated delivery Device. A standard electro-surgical (radiofrequency) cutter creates a 3600 degrees groove in the prostate, a dilatation balloon releases the implant into the groove, and the delivery system is removed. The procedure is completed in minutes and can be performed by any urologist experienced in similar therapeutic procedures.
ProArc’s Technology
The system is comprised of a pre-shaped nitinol implant and an easy-to-use automated handheld delivery device. The unique nitinol implant is anatomically shaped for maximum comfort and efficacy. That together with the automated delivery device creates a much more pleasant experience and simpler procedure for the urologist.
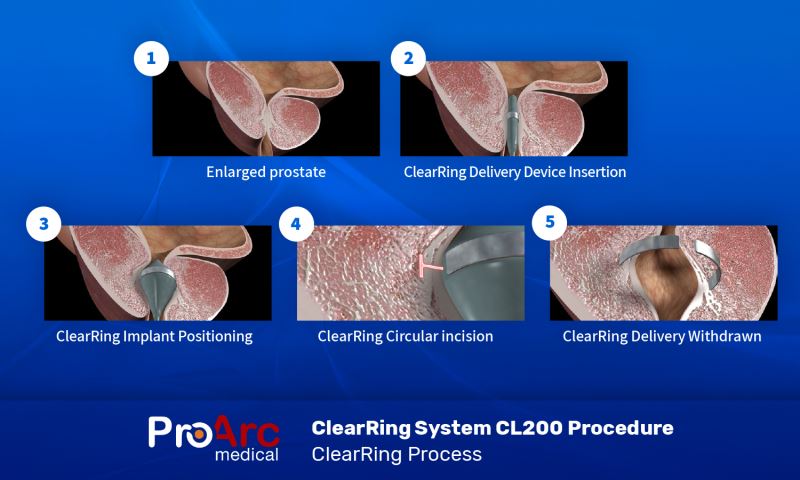
ClearRing Procedure Step-by-Step
- BPH Affect urinary flow and may even obstruct the urethra
- The ClearRing Delivery Device is positioned inside the obstructed urethra
- Implant positioning
- Creating a circumferential groove
- Implant release and Delivery Device withdrawal
Broad IP protection
ProArc has a robust and broad IP portfolio including two granted patents in the U.S., and two granted patents in Europe. ProArc’s patent portfolio gives a broad protection for important aspects concerning the implant, delivery device, and the method of use of the ProArc System.
Moreover, ProArc has additional patent applications pending while continuing to file new applications as new design features are developed.
The technology, IP and the company’s executive management are positioning the company in a favorable position toward successful commercialization and M&A.
ProArc Publications and Citations
ProArc’s solution has been described in published articles in the scientific literature:
- First-in-man Safety and Efficacy of the ClearRing Implant for the Treatment of Benign Prostatic Hyperplasia; September 2018. Urology Focus Journal
- Assessing the Safety and Efficacy of the ClearRing Implant for the Treatment of Benign Prostatic Hyperplasia in a Canine Model; August 2017.Current Urology Journal
- An Update on Minimally Invasive Surgery for Benign Prostatic Hyperplasia: Techniques, Risks, and Efficacy; January 2019
- Current and emerging mechanical minimally invasive therapies for benign prostatic obstruction. Therapeutic Advances in Urology, 2019, Vol. 11: 1–9
ProArc Media Appearances
Prostate reshape implant co ProArc wins $2.2m EU grant, July 2019
Team
Team
|
Robert has spent the past 15 years leading medical technology companies from R&D to commercialization and strategic transactions in roles that included CEO, CFO & VP Business Development. Robert has led companies to M&A with large international healthcare companies. Before that, Robert was a partner at an Israeli venture capital firm. Robert holds degrees in law from New York University and Georgetown University and a BA from the University of Pennsylvania.
|
|
Eli is a Certified Public Accountant (CPA) with a Master’s in Law. Eli has over 20 years of experience as a CFO in privately held and public companies in the NASDAQ, AIM, and TASE (Vocaltec, Cimatron, Ki-Bi mobile, IFN). As a CFO in traded companies, Eli led IPOs and managed financial and operational aspects of international companies, while complying with the regulations of the Securities and Exchange Commission (SEC).
|
|
Founder & owner of Inomec (acquired by Integer) – a Medical Device R&D and production services firm in Israel.
Expert in medical device (minimal invasive delivery systems and surgery tools) development for multi-disciplinary systems and transfer to production including a strong understanding of regulation and QA processes. B.Sc. mechanical engineer from Technion Technological Institute of Israel.
Nowadays, as part of his role at Integer, Sefi leads all the development processes of the ClearRing System.
|
|
Dr. Frank Mastandrea, M.D. is a urology specialist in Tampa, Florida (USA). He currently practices at Florida Urology Partners, LLP, affiliated with St Joseph‘s Hospital of Tampa, FL. Dr. Mastandrea, together with a number of his urologist partners at Florida Urology Partners, invested privately in ProArc.
|
|
Dr. Feld has over 20 years of experience leading innovative research and development projects. He currently is practicing medicine at Rambam Medical Center. He is a serial entrepreneur having founded sever medical technology companies. Yair received his M.D and PhD from the Technion-Israel Institute of Technology.
|
|
Dr. Tay is supporting ProArc in the field of FDA regulatory and clinical affairs. Dr. Tay founded Libra Medical Inc. in January 2007 after 20 years of experience in the medical device industry with specific experience in the BPH industry where she led the regulatory and clinical affairs for NxThera that was acquired by Boston Scientific. She holds an M.S. in chemical engineering, an M.S. in food science, and a Ph.D. in the interdisciplinary area of biomaterials, all from MIT.
|
|
Prof. Ilan Leibovitch is the head of the Department of Urology at Meir Medical Center. He treats renal malignancies, including organ surgery: prostate, testicular, and bladder cancer, including surgery for the reconstruction of the bladder. Prof. Leibovitch is an author of more than a hundred scientific papers, published in leading medical journals and books dedicated to the prostate gland.
|









