Parasonic
292% of funding target
Highlights
Highlights
An innovative and effective solution to the second largest health epidemic in the world
Parasonic offers the first and only solution of its kind to the lice epidemic, the second largest health epidemic in the world, in the form of the first ultrasonic comb, characterized by unprecedented efficiency alongside safety, in a quick and easy treatment. The Parasonic comb is the only solution for the phenomenon of Super-Lice, lice that have developed complete resistance to existing treatments.

A huge global market of $ 1.8 billion a year
Parasonic operates in the global market for lice and lice infestation products, estimated at $ 1.8 billion annually, with sales of chemical products, prevention products, oils and combs totaling hundreds of millions annually in the US alone, and in Western Europe. So far, this market has not yet achieved a technological breakthrough, so the Parasonic comb has no direct competitors, which is expected to help it retain a particularly significant market share.

Registered patents worldwide, approval of sale and first cooperation in the USA
The company’s product and technologies are protected by 3 "families" of patents, in registration procedures worldwide. These patents cover various facets, including application of the technology in the comb itself, the ultrasonic method of operation on which the product is based, and auxiliary accessories. The first patent series has already been approved in the US and Australia in August 2018, and in the next six months registration will be completed in Israel, Canada, Europe and China.
At the same time, the company already holds a sale permit to the US and is in the process of signing a large-scale commercial cooperation agreement, with the largest chain of clinics in the United States for the treatment of lice, including a few hundred branches.

Investing in a product ready for production and distribution, after successful clinical research
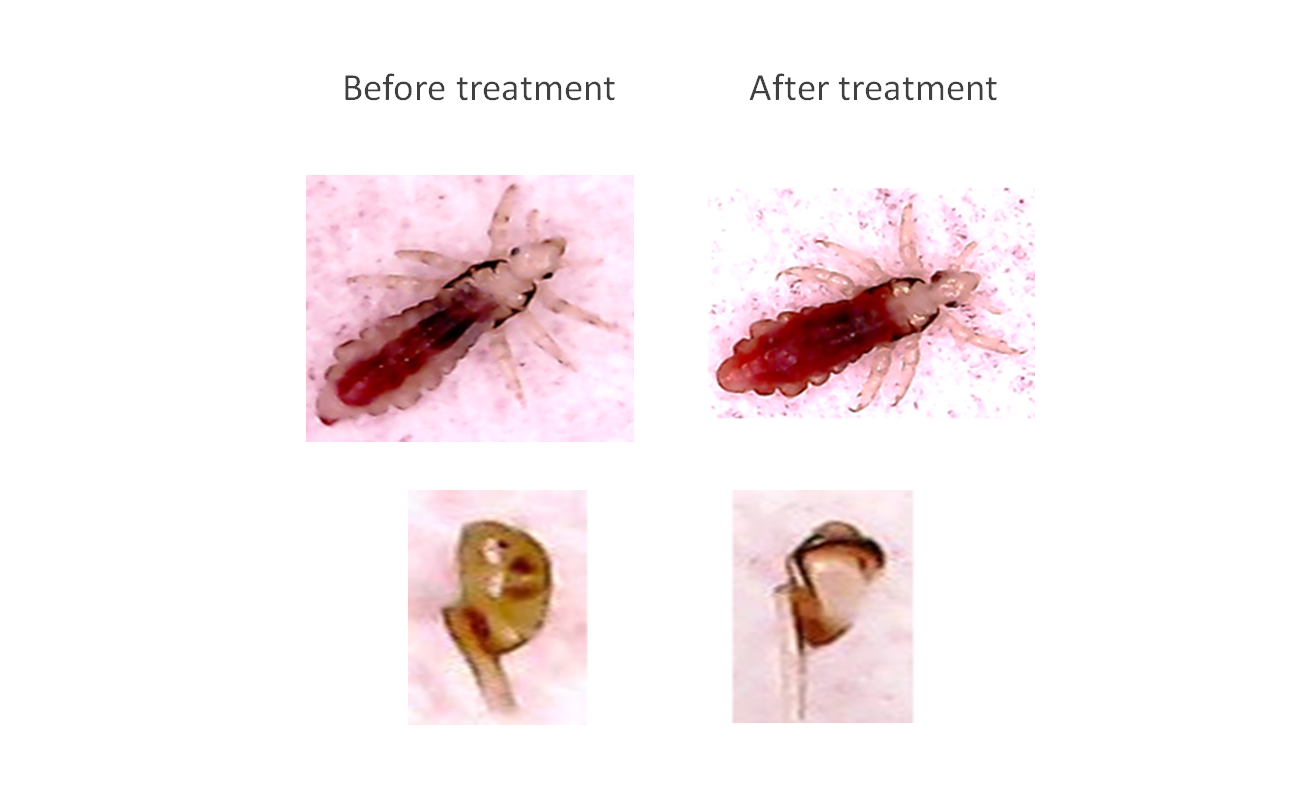
Parasonic's technology is ready for commercial production and marketing after it has been proven to be effective and safe, in a clinical research conducted at the Herzliya Medical Center during 2017-2018. 23 participants were sampled and the procedure was found to be safe and effective, with no adverse effects reported, and over 94% of the lice eradicated. The research data is expected to make the product particularly attractive in a market longing for a solution. The company is expected to be a target for acquisitions for retail, pharma and medical giants due to the great value it creates for them.
Join a leading team that has already raised over $ 3 million
The company's team has extensive experience in development and management, and is led by the company's CEO Omri Ofer, who previously served as CEO of the marketing and sales division of Israel's largest pharmaceutical and healthcare distribution company. This is an opportunity to join an established company after the development and POC stages, which generates a significant disruption in the market in which it operates, having already raised $ 3 million from private investors, including the NGT tech incubator and the Chief Scientist program.
Pitch
Pitch
The Need
Lice is the second largest health issue in the world, after the flu epidemic. However, despite the prevalence of the problem, there has been no technological leap forward in dealing with this problem to this day.
Lice infection occurs mainly in children aged 3-11. In the United States alone, 6-12 million new infections occur each year, with higher infection rates among girls, who usually have longer hair than boys.
For the economy, the lice epidemic means spending over $ 1.8 billion - total cost of OTC treatments, prescription treatments, mechanical combs, preventive treatments, and loss of work days for parents who need to stay home with their children.

A number of key factors reinforce the existing need:
1. Resistance - Lice are more resistant to the chemical treatments available today. In fact, every 2-3 years, lice become resistant to the new chemical products that are released to the market.
2. Efficiency against eggs - Even when the products are effective to some extent against the lice themselves, they are not effective enough against lice eggs. Therefore, treatment has to be repeated after several days to prevent further infection.
3. Side effects - The most common side effects to existing products on the market are skin irritation and allergies, resulting from the exposure of the scalp to these chemicals.
Due to possible side effects of drug lice products, and as a general trend in recent years, the market and consumers are increasingly choosing "natural" products or technological solutions. But the effectiveness of natural products today, made from different plant extracts, has not been clinically proven.
The solution
The lice epidemic refuses to disappear or diminish, and every year infection numbers rise. The phenomenon has been with us since the Stone Age – lice on the heads of human beings are actually modified chimpanzee lice, that has adapted themselves to man.
For a treatment more effective than ever before, ParaSonic developed an ultrasonic comb, which kills lice and lice eggs with a simple combing. In addition, the comb collects the dead head lice into a cartridge, so no rinsing is required after treatment.
Combing with ParaSonic comb enables treatment on a daily basis, which helps to prevent the spreading of lice and egg hatching on the child's head, as soon as they have been infected.
The Technology
ParaSonic's revolutionary comb is based on ultrasound technology and kills lice and eggs in a single combing session, taking about 10 minutes.
The teeth of the comb produce the ultrasound waves directed at the hair that passes between the teeth (this process is protected by the patent), so the lice and eggs on the hair are exposed to these waves and destroyed. The ultrasound waves are gentle pressure waves that operate at a specific frequency, designed to damage only the head and body lice (this frequency range is also protected by a patent).
CarelyTM is a comb with slightly elastic teeth, so the product is suitable for any hair type - smooth, curly or wavy - as well as for any hair thickness.
In the clinical trial conducted among 23 children at the Herzliya Medical Center, the kids reported that the combing was pleasant and expressed a preference for daily combing with CarelyTM, over a weekly combing with the simple, "traditional" comb.
How Does It Work?
Ultrasonic waves pass between the teeth of the comb. The lice and eggs that are found on the hair enter the acoustic area and are exposed to it. Only a second of exposure is needed in order for them to die.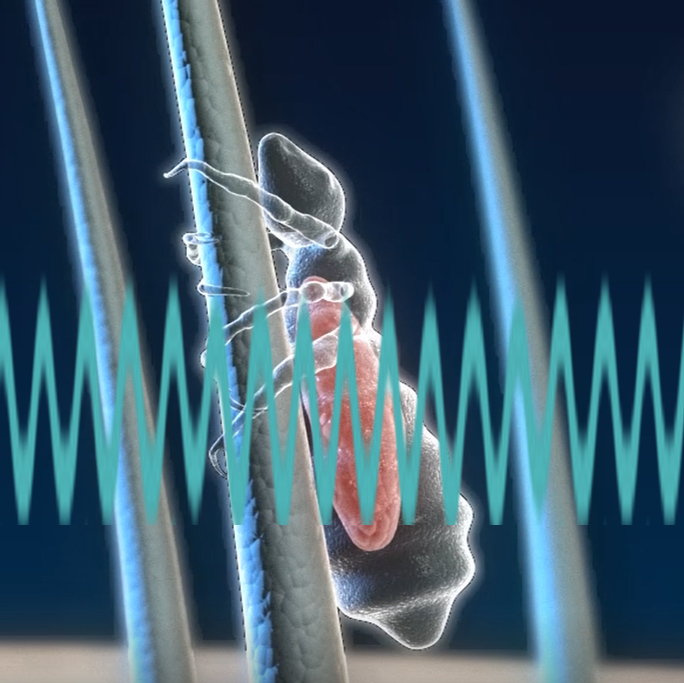
Team
Team
|
Omeri has served as CEO in marketing and sales division over 16 years at Chemipal Ltd. Chemipal is the biggest Israeli company that markets, sells and distributes pharmaceuticals consumer goods and health products with an annual sales scope of approx. 700 million $. He holds an Executive M.B.A. from The Hebrew University.
Majored in Strategic Management for managers of companies.
|
|
Jonathan has over 8 years of experience as R&D and project manager at biomedical startups and ultrasound devices.
He holds a B.Sc in Biomedical Engineering from Ben-Gurion University, and an MBA from the College of Management Academic Studies
|
|
Mor has served as clinical director and product manager at leading Israeli medical esthetics companies. She holds an MSc in Biology/Genetics from Ben-Gurion University, and an MBA from Tel Aviv University.
|
|
Managing director of Jacobs Investment Company LLC, San Diego, CA. Chairman of DermTech International, Nutrinia Ltd., GEO2 Technologies Inc., and director at Fallbrook Technologies.
|
|
CEO and Managing partner of NGT3VC 3, former CEO & director of Beta-O2 Technologies for 8 years. Served as CEO of the Technion Entrepreneurial Incubator for 11 years, led the establishment and investment in more than 50 companies including: Prolor Biotech (sold to OPKO, NYSE: OPK), Mazor Robotics (NASDAQ: MZOR), ReWalk (NASDAQ: RWLK), Corindus (NASDAQ: CVRS), Regentis and many others
|
|
Joe Kalfa is in the Dental Manufacturing and Distribution business, based out of Toronto, Canada.
He is an investor in a number of Dental & Med Tech start ups.
He also is a majority shareholder in a Luxury Consumer goods company based in the USA, that distributes Optical and other lines to companies like Walmart.
|








