NeuroTrigger
the power to blink
111% of funding target


Highlights
Highlights
Breakthrough and patent-pending technology developed due to a personal need
It is rare to witness a case in which a person suffers from an incurable medical situation, but nevertheless initiates and leads to the development of a life-changing solution for himself. Michal Marks, one of NeuroTrigger’s founders, has personally experienced permanent facial paralysis, leading to her inability to close the right eye (to blink), following a head surgery she went through. It is a dangerous situation that causes continuous medical damage and pain, including damage to the cornea, a decline in sight ability until complete blindness, difficulty in exposure to light, severe headaches, and loss of function and concentration. Inspired by Michal and led by her, NeuroTrigger was founded and developed the first blinking pacemaker worldwide. This life-changing device is based on an electrical stimulator unit, electrodes, and a controlling mobile app. The device is set on the ear, like a hearing aid, enabling personalized, pain-free, practical, and daily use.

Significant grants on behalf of the IIA, winning lucrative awards and strategic partnerships
Thanks to its technological and medical innovation, NeuroTrigger has won several significant development grants on behalf of the IIA at a total sum of hundreds of thousands of dollars. Several months ago the company has successfully completed its first clinical trial with the Reconstructive and Aesthetic Surgery (Plastic Surgery) department in Tel Aviv Sourasky Medical Center Ichilov. Moreover, during 2019 the company has won the first prize in the lucrative startup competition Merage 45+, including a significant financial award and access to Merage’s lucrative program in California, dealing with penetration to the U.S. market, meetings with investors, and leaders of the global medical device industry.

The first-of-its-kind solution for an extremely vast market
NeuroTrigger developed a life-changing medical device that provides a solution for hundreds of thousands of people that experience each year temporary or permanent facial paralysis. This market is evaluated by Billions of dollars per year, and in fact, facial paralysis is much more common than we think, and across all ages, as just recently the public in Israel was exposed to several cases of Bell’s palsy among several celebrities such as Michal Ansky and the model Anna Zack.

A high-quality team composed of world-class engineers and physicians.
The company’s founding team holds a multidisciplinary business and developmental experience, with significant experience in leading technological companies. The company’s CEO, adv. Michal Marks has gained years of experience in facing facial paralysis due to her personal experience. Alongside Michal, the founders, Dr. Asaf Deutch and Shachar Paz are seasoned engineers with an accumulative experience of dozens of years in engineering and management. For example, Shachar has led multidisciplinary teams in leading engineering companies such as Elbit and Applied Materials, while Asaf has led the planning and development of FDA-approved products, in addition to patent registration in the bio-medical field. The company is accompanied by Prof. Eyal Gur, the director of Reconstructive and Aesthetic Surgery (Plastic Surgery) in Tel Aviv Sourasky Medical Center Ichilov and a world-class surgeon in facial reanimation, as the company's medical director, and Professor Anat Levinstien, a world-class ophthalmologist and the manager of Tel Aviv Sourasky Medical Center Ichilov ophthalmology division.

Pitch
Pitch
Facial paralysis can be caused by a variety of reasons, including trauma, a side effect of head surgery, Bell’s palsy, etc. Most people with facial paralysis cannot close their eyes on the paralyzed side and blink like any other healthy person. This problem causes severe phenomena such as extreme dryness in the eye, headaches, tiredness, extreme sensitivity to light, damage to the cornea, significant damage to the quality of life, and even loss of sight in the paralyzed eye. Currently, all the existing solutions in the market such as eyedrops treat only the symptoms, but none of them restore the natural blinking ability.
NeuroTrigger was founded following a facial paralysis of its CEO, Michal Marks, after a head surgery she went through to remove a tumor. After several years in which Michal unsuccessfully searched for different solutions to restore her blinking ability in the paralyzed eye, she founded NeuroTrigger with a team of friends who are experts in electronics and medical devices development. Together, they developed the first personal blinking pacemaker in the world, especially for Michal. Michal is the first person worldwide to use the prototype developed for her on a daily basis, and her quality of life has significantly improved as all the side effects she experienced due to the inability to blink are gone.
The solution
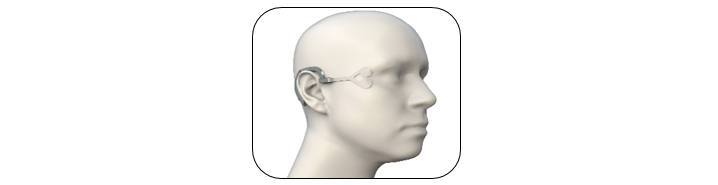
NeuroTrigger developed BlinkER – the first blinking pacemaker in the world. The system performs what was unthinkable and retrieves motor activity to a paralyzed organ hence restoring the natural blinking activity. The product is composed of a personal charging unit that generates a unique electrical pulse, to which a finely designed and disposable electrode is connected. The electrode transmits the electrical pulse to the nerve and the muscle which are responsible for blinking. The system connects to the mobile phone via a designated app and the user can select different parameters such as the desired blinking pace, the intensity of the electrical pulse, etc. The BlinkER system is easily operated, charged, portable and reliable. The pulse generator is located behind the ear resembling a small hearing aid, while the electrodes are placed next to the eye, externally. The system creates an advanced, elegant, and high-quality solution that enables the paralyzed eye to blink properly.

The technology
NeuroTrigger has developed the first pacemaker in the world, which is composed of the following components:
- A suave signal generator is placed on the ear similar to a hearing aid. It generates an electrical pulse and transmits it to the electrode, and it is rechargeable and lightweight.
- A unique electrode, daily and disposable, connects magnetically to the signal's generator. The role of the electrode is to precisely and painlessly transmit the electrical pulse to the nerve and the muscle which are responsible for the blinking activity. The electrode has a minimal and slick design, so it looks more like an accessory rather than a medical product.
- A mobile app that enables the user to control the pace and the intensity of the electrical pulse.
Reusable Neurostimulator
- Portable
- Rechargeable
- User friendly
- Aesthetic
- Lightweight

Daily-use Electrodes
- Disposable
- Biocompatible
- Easily attached
- Minimally designed.
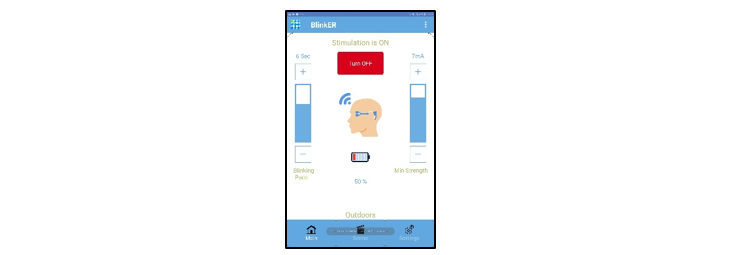
Mobile App
- On/Off
- Blink pace control
- Pulse intensity
- Cloud diagnostics
The innovative and groundbreaking patent-pending technology developed by NeuroTrigger was approved as PCT and afterward, it was filed for the national phase in The U.S., Europe, China, and Israel. In addition, the company is working on filing additional patent applications, for patents that were created during the R&D process. Besides the patents that are processed, another important milestone in the clinical trial held by the company as part of its partnership with the Reconstructive and Aesthetic Surgery (Plastic Surgery) department in Tel Aviv Sourasky Medical Center Ichilov. The prototype developed was successfully examined on 20 patients that suffer from facial paralysis due to various reasons. The excellent results received, strengthen the confidence of the company in its product and will assist the company with the product’s approval process in international markets.
Moreover, the company is considering an additional future development of products such as a permanent electrode implant, a technological development that will enable synchronized blinking of both eyes (the healthy and the paralyzed), an adapted product for ventilated patients in ICUs and additional products that will provide medical treatment for additional eyes problems that constitute a tremendous global market, such as treatment of a dry eye due to deficient blinking among Parkinson and Alzheimer patients, and neurological treatment for the dry eye syndrome.
Team
Team
|
Mechanical and Bio-Medical Engineer, complemented with PhD in Biophysics, from BIU. Experience of 27 years in development and manufacturing of medical devices and research instrumentation. Served as the engineering manager of multiple projects for planning and development of FDA approved products. Co-Founder of Prizmatix Ltd. and Lumiworks-Photonics Ltd. that develop and export electrooptical systems for research and industrial use. In addition, he has registered multiple patents in the biomedical field and has published several articles in the biomedical field.
|
|
Graduated from Haddasa medical school, HUJI, and an obstetrics and gynecology specialist.
Holds vast experience of more than 20 years as a physician in hospitals and in community clinics. Managed and participated in multiple clinical trials in various settings. Recently appointed as assistant medical director in central district of Clalit medical services.
|
|
Physicist and Electronics Engineer graduate from TAU with an M.Sc. in physical electronics from TAU and BIU. Shachar holds more than 20 years of R&D experience of cutting-edge products in inspection, Semiconductor & multi-disciplinary systems in metallurgy, microscopic, 3D imaging and computerized imaging and drones from Nova (Nasdaq: NVMI), Applied Materials (AMAT), Sarine tech and Elbit systems. In addition, Shachar has registered numerous patents in metallurgy and in the biomedical field and has published several articles.
|
|
Head of Plastic & Reconstructive Surgery Department, Tel-Aviv Sourasky Medical Center (Ichilov); Clinical Professor, Faculty of Medicine Tel-Aviv
University; Past Chairman Israeli Society of Plastic & Aesthetic Surgery.
|
|
Chair of the Department of Ophthalmology, Tel Aviv Sourasky Medical Centre (TASMC), Professor of Ophthalmology and Vice Dean, Tel Aviv University, Israel.
|





