Earways Medical
119% of funding target
Highlights
Highlights
1. Cutting Edge Technology
“Simplicity is the ultimate sophistication”
*Leonardo da Vinci
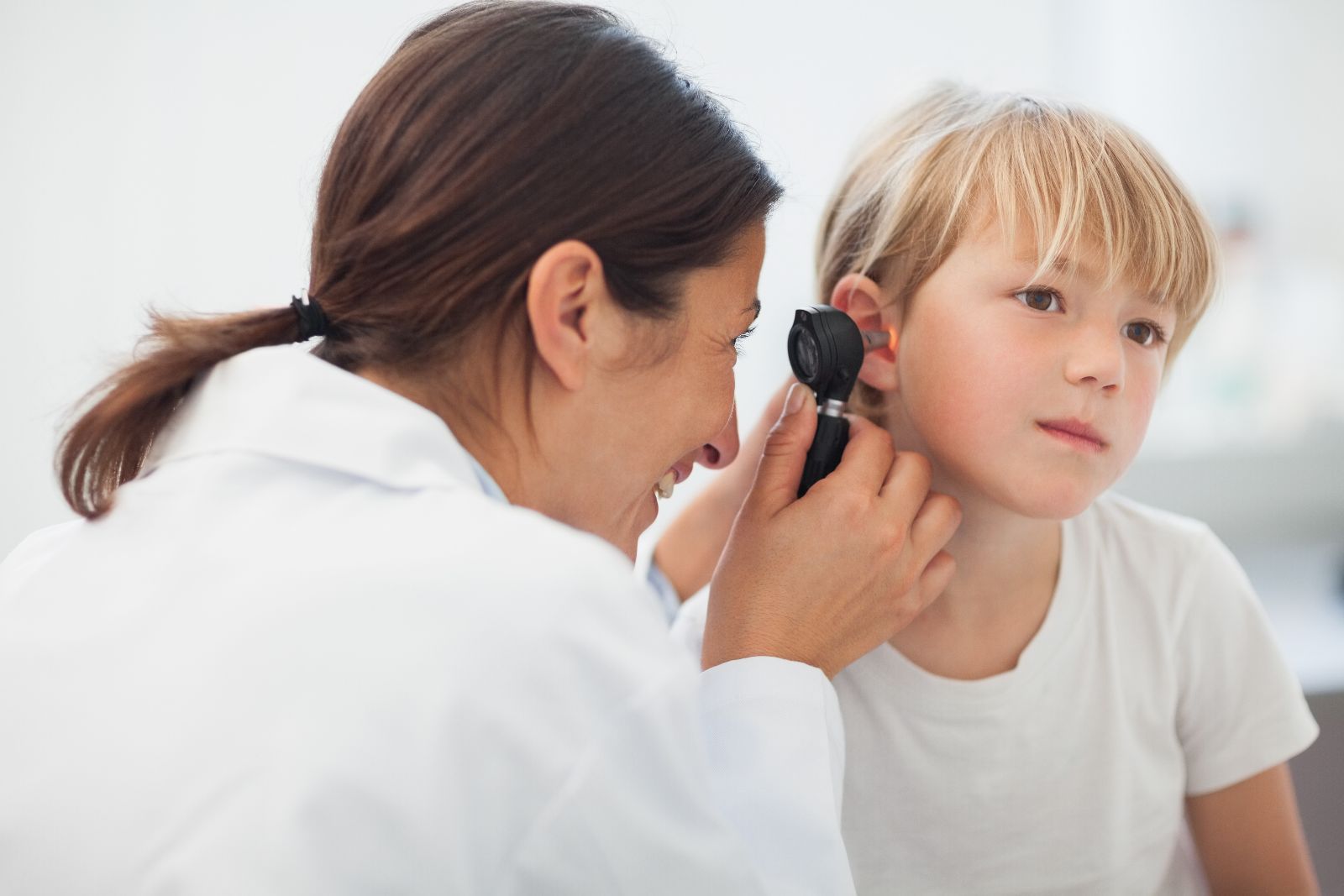
Earwax blocking the ear canal, also known as cerumen impaction, is the top treatable cause of hearing loss. This common phenomenon, which affects 1,12 Billion people worldwide, can also cause earache, dizziness and discomfort.
In the past 30 years, there has been little to no innovation in the field of earwax removal.
EARWAYS MEDICAL offers a breakthrough solution to the problem.
The EarWay™ products are based on unique patent-pending technology. The innovative design enables simple removal of earwax from the ear canal. The flexible, helix-shaped tip manoeuvres along the walls of the ear canal, gathering cerumen and extracting it in one cluster.
Proven in clinical studies, EarWays’ technology ensures safe and effective cerumen extraction, without harming the ear canal or eardrum. When untreated, earwax impaction may lead to loss of hearing, which can affect cognitive and social capabilities.
2. Joining the EARWAYS Path to Success
The founders of EARWAYS identified a true market opportunity that answers an unmet need. Following their vision and remaining loyal to their strategy, EARWAYS MEDICAL have successfully accomplished clinical studies that generated solid clinical evidence. EARWAYS MEDICAL achieved significant milestones enabling fast acceleration of research and development, as well as strong channels to market.
EARWAYS is supported by the Israeli Innovation Authority (formerly known as the Office of the Chief Scientist) and it is endorsed by Israeli and European Key Opinion Leaders (KOLs).
The Company has ISO certification for medical devices.
From a commercial perspective, the Company has already obtained the CE mark permitting sales around the EU and other countries. In addition, EARWAYS has obtained Israel's Ministry of Health approval and FDA clearance is expected by the end of 2018. Commercial distribution agreements and activity in the UK, Scandinavia, France, Belgium and Poland will ensure rapid growth and scale-up opportunities.
3. Winning Team
Boosted by an inherent medical demand and backed by clinical evidence, the Company benefits from a team of world-class experts in bio-engineering, medical devices, ear surgery, hearing loss and rehabilitation. Together with its experienced entrepreneurs who have vast business and marketing knowhow, EARWAYS MEDICAL is positioned to transform accessible ear care.
4. Breakthrough Product
EARWAYS MEDICAL’s products are disruptive and provide a breakthrough in the management and treatment of earwax (cerumen).
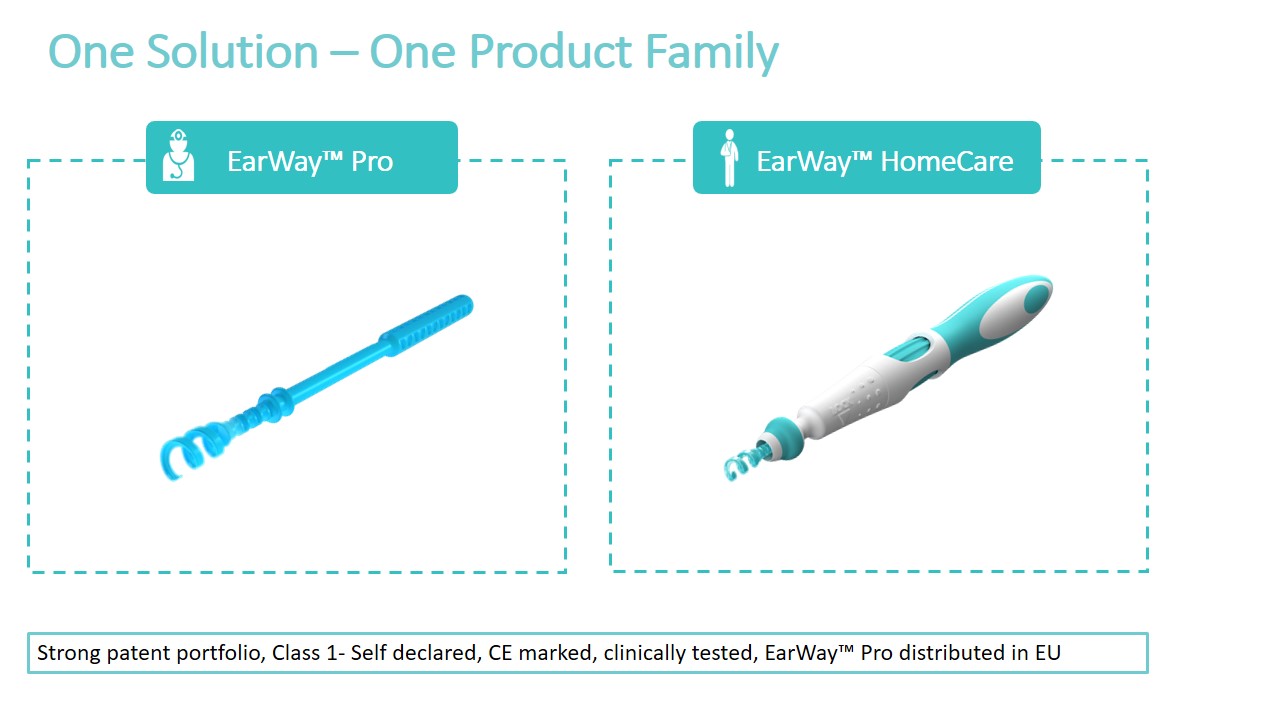
The Company offers 2 products aimed at 2 markets: The professional health care market and the consumer market (OTC). The main benefit of both is the fast extraction of earwax from the ear canal, providing the patient with immediate, effective and safe relief!
Pitch
Pitch
|
EARWAYS MEDICAL is a leading innovator of ear-care solutions. Founded to enhance the well-being of millions of children and adults worldwide, EARWAYS MEDICAL provides easy-to-use ear care products designed for clinicians and home use.
THE PROBLEMEarwax lubricates, cleans, and protects the lining of the ear canal by repelling water, trapping dirt, and ensuring that insects, fungi, and bacteria do not get through and harm the eardrum. Earwax, also known as cerumen, is a yellowish substance that is produced by the sebaceous gland within the ear. Cerumen can be found in a number of forms, ranging from liquid, through soft to hard earwax, depending on genetics as well as stress, fear and environmental and climate factors.
The human body has a self-cleaning mechanism that utilizes jaw movements to drive excess ear wax through the ear canal and out of the ear. When this mechanism does not function as it should, there is a need for external intervention to routinely and properly remove the excessive earwax. Earwax clogging is more common in warm climates, and is intensified by frequent use of headsets and ear plugs, which prevent ventilation of the ear canal and stimulate earwax production. Cerumen impaction is the number one cause of various degrees of temporary, treatable hearing loss. Many people are often unaware of partial loss of hearing, which has a direct effect on quality of life, including productivity, frustration, behavioral changes, and even social withdrawal. In some cases, impacted earwax can cause earache, and in rare cases tinnitus (ringing in the ear) dizziness, and vertigo (loss of balance).
Hearing Aids and Earwax – a Challenging Combination People wearing hearing aids often complain of an increase in earwax. The reason is that hearing aids may stimulate the glands producing earwax. In fact, earwax blocks 60-80% of hearing aids’ filters, resulting in reduced efficacy of the device. Thus, the objective of improved hearing is not always achieved. In addition, the hearing aids themselves, located in the ear canal, also block the natural passage of excess earwax, out of the ear. The American Academy of Otolaryngology – Head and Neck Surgery recommend that if you are prone to repeated wax impaction or use hearing aids, you should carry out routine preventive cleaning. It is also important to maintain clean hearing aids.
THE SOLUTIONThe novel EarWay™ products, based on innovative patent-pending technology, are designed to solve the challenge of earwax blockage. EarWay™ Pro for healthcare professionals and EarWay™ HomeCare for self-removal, are devised to enable simple extraction and management of earwax accumulation and impaction. The solutions have been clinically tested and are proven safe and effective.
|
THE TECHNOLOGY
EarWayTM Pro - Professional Product
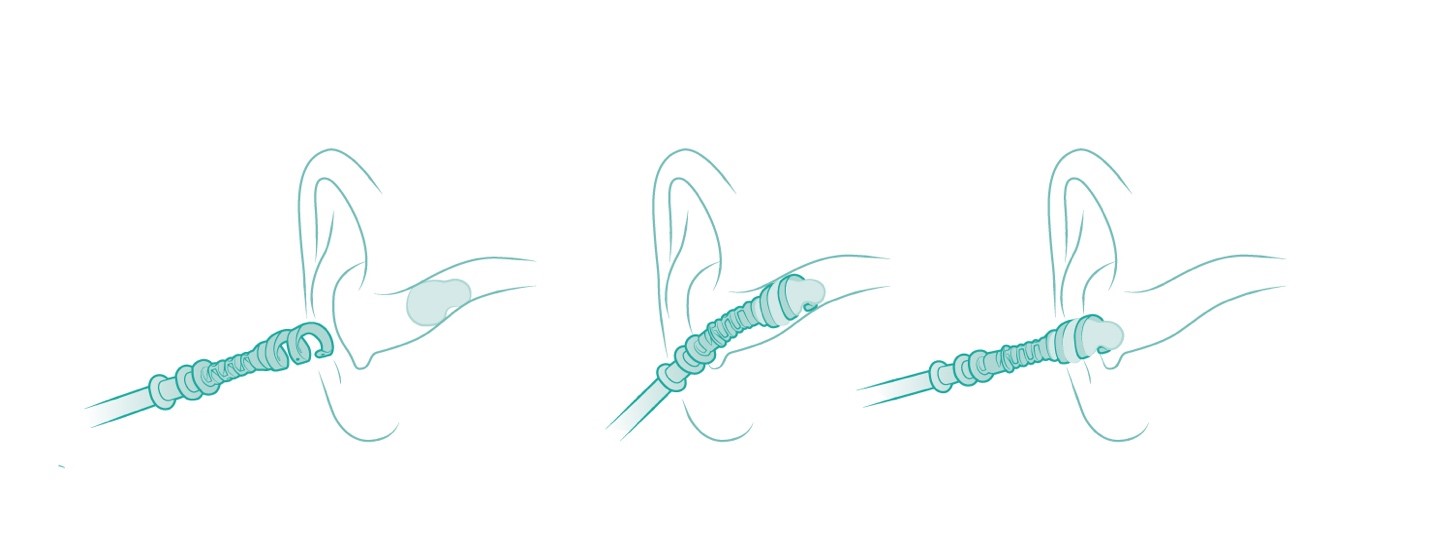
Based on patent-pending disruptive technology, the EarWay™ Pro device is designed with a flexible helical tip, for easy navigation in the ear canal. This single-use product is rotated inward into the ear, collecting the cerumen, and extracting it from the ear canal as a single cluster.
Animation Movie EarWay™ Pro
- Simple procedure - EarWay™ Pro delivers a fast, efficient and clean solution designed to simplify and even save the high skill and expensive equipment required for currently used methods.
- Saves "chair" time - EarWay™ Pro shortens the duration of the procedure, which can take even up to half an hour per ear with existing products. This benefits healthcare providers and patients alike.
- All healthcare providers - Pediatricians, general practitioners, nurses and audiologists are not as skilled as Ear, Nose and Throat (ENT) doctors, and lack the highly expensive visualization equipment used until now. EarWay™ Pro is a simple and inexpensive solution for them. Ear Nose and Throat specialists have referred to EarWay™ Pro as the “safest, most effective product in market for non-ENTs”.
- Reduces burden on health care system - EarWay™ Pro can extend the scope of service providers to audiologists, pediatricians, geriatricians, nurses, emergency room nurses and nurses in nursing homes, thereby reducing the burden of the health system and alleviating the patient's suffering.
- Cost effectiveness - Since the extraction time of cerumen with the EarWay™ Pro is shorter than the time required when using existing instruments, we estimate that doctors can perform more procedures and attend to more patients.
- Prevents cross-contamination – Ear wax carries spores of infectious and contagious disease as well as fungus. EarWay™ Pro is a single-patient, single-use device, which eliminates the costs related to sterilization of products, as well as preventing cross contamination.
EarWayTM HomeCare - consumer product
Release with a Twist
The EarWayTM HomeCare device is based on the same clinically proven technology as EarWayTM Pro. It is designed for home use, either independently or by a family member or carer. With an easy “release with a twist” mechanism, the EarWayTM HomeCare has a dial that enables the user to insert the flexible tip and reach the appropriate place. When the device enters the ear, it collects the earwax. The swivel dial will ensure the tip stops at a pre-defined distance from the eardrum. When removing the device, the earwax is withdrawn in a single cluster. Each EarWayTM HomeCare helix tip is intended for one-time, single-use, to ensure hygiene.
Removal of the clogging earwax should provide immediate relief. In cases where symptoms continue, a patient should seek medical advice.
The EarWayTM HomeCare device will be commercially available during 2018.
Animation Movie EarWay Home Care:
Team
Team
|
Dr. Amir Kraitzer is a biomaterials specialist, entrepreneur, and inventor in the field of medical devices. He has a proven track record in management and problem-solving in both industrial and scientific environments.
Dr. Kraitzer has acquired vast expertise in engineering polymeric materials for medical use, bio-resorbable polymers and cements, biomaterials, controlled drug release and tissue engineering. He also has broad experience in medical device technology, product development, manufacturing and commercialization, and compliance with regulatory requirements – both in the U.S. and the Europe.
Previously, he was a co-founder and CEO of Augma – a biomaterials company which developed biodegradable cement-based bone graft substitutes.
Amir holds a PhD, Bio-Medical Engineering; MBA, Strategy and entrepreneurship; MSc, Materials Science and Engineering – Biomaterials and implantable medical devices, graduated cum laude, and BSc, Mechanical Engineering Energy, Control and Robotics – all from the Tel-Aviv University, Israel.
|
|
Ms. Yael Karlin has extensive international experience in the start-up ecosystem with a comprehensive knowledge of the life science industry. Since 2008, she has advised and coached start-ups on strategy, business development, and raising capital.
Ms. Karlin excels at creating partnerships, generating R&D and licensing agreements, commercialisation, joint ventures, and acquisitions.
She has held sales and marketing positions at Cordis, and at Johnson & Johnson – both in Israel and in Belgium, and led the marketing activity for a US-based start-up. Prior to that, she directed the Start-up Centre at the Israeli Export Institute, and filled a technology matchmaking position at Ernst & Young Israel.
Yael holds an MBA and a BA in Management from Ben Gurion University, Israel, and is fluent in English, French and Hebrew.
|
|
Dr. Udi Katzenell is a specialist in Otolaryngology, and Head of the Otology Service and the Cochlear Implant Program at the Department of Otolaryngology, Head and Neck Surgery at the Kaplan Medical Center in Rehovot – affiliated with the Faculty of Medicine of the Hebrew University of Jerusalem.
His scope of interest is the treatment of ear diseases and hearing rehabilitation of people with hearing loss and deafness. Dr. Katzenell has practiced medicine since 1995, and spent two years in Auckland New Zealand and Melbourne, Australia, practicing ear surgery and hearing rehabilitation as a fellow. He is a lecturer at the faculty of Medicine of the Hebrew University, and lectures on ear surgery at international conferences. He has also published numerous professional papers.
|
|
Dr. Ohad Hilly is a Head and Neck surgeon, and is Head of Ear Surgery in the Ear, Nose and Throat Department at the Rabin Medical Center affiliated with Tel Aviv University.
Having received his medical degree at the Sackler School of Medicine at Tel Aviv University, and after completing his internship at the Ear, Nose and Throat Department at the Rabin Medical Center, Dr. Hilly completed his specialization in ear surgery, neuro-otology, and skull-base surgery at the University of Toronto, Ontario, Canada. He is a member of the Israeli Medical Association; Israel’s Ear, Nose, Throat, and Head and Neck Surgery Association; and the Israel Neurological Association.
Dr. Hilly’s special fields of interest include: surgery for restoring hearing loss: cochlear implants; middle-ear implants; bone conduction implants; skull-base surgery; and acoustic neuroma. His research areas are: head and neck cancers, cochlear implants, and imaging of the cerebellopontine angle.
|
|
Mr. Lambert is a highly experienced audiologist and hearing-aid specialist, who has treated diverse hearing-related problems, including deafness and tinnitus.
Mr. Lambert is the owner of Audition Comfort SPRL – which he founded in Brussels in 2007. The company distributes hearing aids and hearing protection accessories, providing patients with the latest and most innovative solutions for hearing rehabilitation. Mr. Lambert is also active in many university hospitals in Brussels and its surroundings.
He joined the team after recognising the importance of EARWAYS MEDICAL’s products for patients using hearing aids and accessories. Mr. Lambert studied at Libre Marie-Haps 3 University, adiologue-audioproth?siste and at the Solvay entrepreneur program.
|