CoreBone
316% of funding target
Highlights
Highlights
A Revolutionary Breakthrough Which Is Bioactive, Strong And Remodels
The breakthrough development achieved by CoreBone relates to many of us who may need bone grafts now or in the future. CoreBone’s bone graft gives surgeons — and patients — an alternative to "spare parts" sourced from cadavers, either human or animal, and the option of adopting a new, natural and low- risk solution.
Most bone graft materials currently available in the market are derived from human and animal bone. Human products carry the risk of disease and rejection and face significant regulatory burdens in many countries. Animal bone products from cows or pigs (bovine or porcine) do nor resorb, which means they don't grow and aren't replaced by new bone, due to the heating process they undergo to eliminate viruses. Synthetic bone graft materials, on the other hand, lack strength.
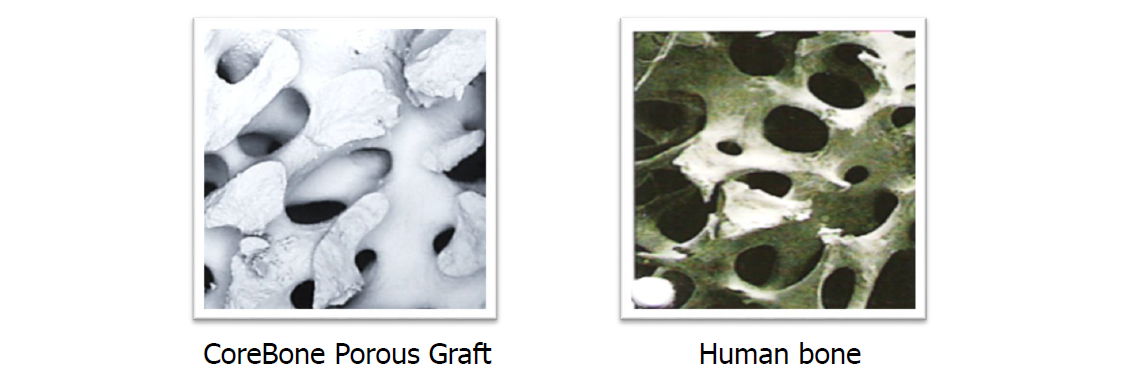
CoreBone leverages natural bonelike qualities and presents a strong, bioactive, low-risk product, as well as a natural and environmentally friendly solution.
Patent-Protected Technology
CoreBone grafts are made from corals that are grown and cultured in the closed, monitored laboratory environment of its coral farm. The company carefully selected and tested special coral types to ensure suitability for bone grafting and efficient, cost-effective coral cultivation.
Patented technology (issued patents in the US and Israel; EU in process) embeds bioactive minerals (silicium) into the skeleton of selected coral types during the growth process, utilizing their bonelike qualities and adding bioactivity for accelerated healing and better bone connectivity. As a result, the CoreBone graft material offers the same bonelike qualities as organic material (human-derived) without the associated biological risks, and also grows at a rapid rate, over 10 times faster than usual.
Funding To Date
CoreBone has received the support and funding of the Israel Innovation Authority (previously called Israel’s Office of the Chief Scientist) to develop its innovative solution. Among its investors are The Trendlines Group, a leading Israeli investor in early-stage medical devices and agricultural technologies. Trendlines was the most active (by the number of investments) Israeli life science seed stage investor in 2016. Additional investors in CoreBone are the founders and angels from Israel and abroad.
Novel Solution for a $4 Billion Market
The global bone graft market for the orthopedics and dental fields is estimated at $4 billion. It is growing as the global population ages and seeks better aesthetics and greater functionality. The dental market is growing at 11% annually as result of dental implant growth globally. Animal-derived bone leads the dental market. Bio Oss (Geistlisch), the cow bone-derived graft, is the sector’s leading product, with 25% market share. The orthopedics market, which represents 75% of the total bone graft market, is more mature and growing at 4.5%. Leading product categories in orthopedics are bone grafts derived from human bone.
Most people who undergo bone grafting procedures are unaware of the source of the graft: human cadaver or animal. CoreBone’s new, natural, efficient and cost-effective graft material offers an effective alternative to these products.
CoreBone plans to penetrate selected markets, acquire significant market share and establish a natural and effective solution for the current market leaders.

Already Raising Interest and Excitement in the Market
Since its launch early this year, users and leading KOLs in the dental market have already given favorable reviews after using CoreBone, supporting the CoreBone system as a natural alternative to current cow (bovine)-derived products.
It offers a favorable solution to the growing ethically conscious, vegans and religiously observant segments. It also offers competitive pricing, about 30% to 40% below the price of market leaders.
Human-derived products are not commonly used in Europe due to regulatory burdens and public sentiments. Animal bone (bovine, porcine) are treated at high heat (some 1,000°C) to eliminate dangerous viruses such as mad cow disease. Such treatment prevents remodeling (replacement of the graft by the new bone). This is the main reason for the very limited use of animal bone in orthopedics.
CoreBone’s graft remodels as bone grows, enhances new bone cell attachment and the creation of new bone due to its novel, patent-protected development.
The World Is Ready for CoreBone
Israel is regarded as a world innovation leader in the dental field. Several dental companies have been acquired by global leaders for substantial amounts.
CoreBone’s unique and innovative product, developed in Israel, commenced its market entry into the dental field. Launched at the International Dental Show in March 2017, the CoreBone graft has already produced sales in the tens of thousands of dollars -- and is expected to grow to the hundreds of thousands in 2018.

* It was found that CoreBone's product is 5 times stronger than the tested human bone.
Regulator-Approved and Ready for Marketing
CoreBone has regulatory approvals to market its products for dental and orthopedic indications in Europe (CE mark) and Israel. It is already marketed in the dental field, mainly in Israel and Poland; the company is in discussion with dental implant leaders and local distributors about entering new markets in Europe, South and Central America and Asia.
CoreBone is in the process of fund-raising to expand its activities, enter new markets, generate greater sales and gain market traction, including FDA 510(k) submission approval in the US, an orthopedic clinical study and extensive marketing activities.
Pitch
Pitch
Rising environmental awareness around the world and the need for green, natural solutions has made an impact in medicine, resulting in a growing demand for friendly and ethical products. That has been at the background of CoreBone’s development, as current bone graft solutions are derived from human or animal bone, with their negative characteristics and associations.
Corals are known to make good bone graft material as they have similar physical and chemical structures to human bone. Indeed, coral-derived grafts have been in use for over 20 years in dentistry and orthopedics. However, these products are derived from the sea, prone to marine contamination and are not monitored over their growth conditions. Moreover, the Kyoto agreement declared coral as an endangered species and banned coral harvesting and trading.
How Does It Work?
CoreBone grows its coral in a closed, monitored environment using distilled drinking water to create laboratory-made enriched seawater that not only adds bioactivity but prevents potential biological and chemical contaminants.

Located in Israel’s Arava Desert, and far removed from any external marine environment, the CoreBone laboratory uses solar energy, among other methods, to successfully grow, harvest and process coral into medical products.
CoreBone's Advantages

Competitor Comparison


Our Next Goals
Forecast - subject to updates

The Business Model
Typically, bone grafts are sold to dental and orthopedic surgeons, clinics and hospitals by medical device (implant) distributors or by the medical device companies (manufacturers) directly.
Our business strategy is based on three modular phases: initiating our sales in the dental market via local global distributors and directly; signing distribution agreements with global strategic dental players after we reach some traction in the market; entering the orthopedic field.
Stage 1: Sales
CoreBone markets products online and through distributors (implant companies and medical equipment companies). The company currently operates with three active distributors in the dental field: two Israeli companies and another Polish company. CoreBone is about to sign a significant distribution agreement with a leading company in Romania. In addition, the company has received numerous distribution requests for its products from leading distribution companies in France, the Czech Republic, India, Taiwan, Mexico and Argentina. There are a number of other countries in the process of being examined as well.
Stage 2: Cooperation with implant companies
CoreBone will sign global cooperation agreements directly with implant companies, is in initial negotiations with several leading bodies already underway, and has a first cooperation agreement already bearing fruit.
In addition, the company will start selling raw materials it manufactures -- coral for final processing -- in countries in emerging markets such as China and India.
Stage 3: The orthopedic market
CoreBone holds regulatory approvals to market its orthopedic products in Europe and Israel. At the same time, this market is more institutionalized than the dental market and is controlled by global companies. In order to penetrate with a new product into the orthopedic market, institutionalized clinical research and collaboration with leading orthopedic agents is required. CoreBone has already received several requests from orthopedic distributors in Europe and India.
CoreBone also began planning a clinical research at the Sourasky Medical Center (Ichilov), a study that will progress at the end of the induction. Another study using a European CRO (clinical trial management body) is in the planning stages. Initial results of the study are expected to be received approximately six months from commencement. Success in the clinical trial will advance CoreBone's orthopedic product, in a significant step towards commercial scale production and marketing.
CoreBone has already signed a confidentiality agreement with several leading global orthopedic companies.
Team
Team
|
Internationally recognized researcher in bone biology and dentistry. Previously, he served as the head of the Dental Dept. and Hard Tissue Laboratory at Sourasky Medical Center in Tel Aviv and at Tel Aviv University’s School of Dental Medicine and Dept. of Bioengineering. He was a senior researcher at the Hospital for Special Surgery in New York and a medical advisor to Interpore ProOsteon, the first coral-based bone graft (later sold to Biomet for $270 million). He has authored or co-authored more than 100 publications in dentistry and bone regeneration.
|
|
A genuine expert with vast experience in raising coral, Assaf established a coral farm at Ein Yahav, in Israel’s Arava Desert, as an alternative to traditional the agriculture farms that export produce to Europe but now face fierce competition from countries with where labour costs are considerably less costly. Assaf joined CoreBone in its early days to manage the activities related to coral growing and development. Previously, Assaf built complex lighting projects, Yad Vashem Holocaust Museum among them.
|








