ADVA Biotechnology
176% of funding target


Highlights
Highlights
The long awaited revolution in cancer treatments
Cell therapies, a new and clinically effective cancer can change the way modern medicine fights cancer. Unfortunately it is dramatically limited due to high cost (about $500,000 per patient) and labor intensity as well as manufacturing limitations. Adva Biotechnology enables personalized lifesaving therapies in a decentralized automatic manufacturing model, allowing treatment on site in hospitals or near patients. This allows reduction of logistic burden and costs, simplifying the process and ensuring availability.

Unprecedented clinical efficacy allows the company to ride the wave of regulatory change
Since these advanced therapies such as CAR T, TCR, NK and TIL’s ,have proven highly effective in treatment of cancer with over 80% efficacy of end stage patients, they have received unprecedented regulatory approval by global regulatory agencies such as the US FDA and the Israeli MoH. This clinical efficacy allows companies to sell to the market after phase 2 studies only, bypassing many regulatory setbacks, making its market entry easier and faster, for the benefit of share-holders and patients alike.

Ongoing negotiations with leading market players
The company and its breakthrough product have gained traction in the market, thanks to the solution it offers in regards to easy manufacturing, solving a major issue in availability of the therapy to patients for many Big Pharma giants. Accordingly, several large companies and manufacturers in the field are already in contact with the company, with initial cooperation and use of the technology, including some of leading names in the field, as well as leading Israeli and US hospitals and universities. The company is in its final stages before formal global launch.
Unique technological IP and worldwide patents
This far company has filed for the registration of several patent applications to the USPTO on the designs, control schemes, algorithms, methods, and physical elements which will be followed by worldwide acceptance. The original patent filed in 2016 is now being examined in different regions of the world covering over 33 unique claims.
Join the future market leader, seconds before take-off
The main benefit for investors joining forces with Adva Biotechnology at this moment, is the greatly advanced state the company is currently in, with a product nearly ready for market, initial customers, and a large pool of possible income revenues and interested field players. This represents a real one-time opportunity for private investors to join the company and enjoy the benefits and success of its revolutionary technology that can not only bring financial gain but save lives.

Pitch
Pitch
The Problem
Cancer is a widespread global medical issue which is well-known to many people, whether from self-experience, or through the suffering of loved ones.
According to the National Cancer Institute (NCI)cancer is already the second leading cause of death globally. The number of new cancer cases in the world per year is expected to rise to 23.6 million by 2030, and according to the world health organization, it is, accounting for 9.6 million deaths in 2018 alone.
The widespread nature of this disease has major financial implications. The American National Institute of Health (NIH) reported that the national expenditures for cancer care in the US totaled nearly $125 Billion in 2010, and could reach $156 Billion in 2020. The number of new cancer cases is expected to rise from 18 million to 23.6 million within the next two decades up to 2030. It is estimated that the US national expenditure on Lymphoma alone is 16-20 Billion USD per year.
Traditionally, for many years, the pillars of cancer treatment have been surgery, chemotherapy, and radiation therapy. Over the last two decades, targeted therapies like imatinib (Gleevec®) and trastuzumab (Herceptin®), drugs that target cancer cells by homing in on specific molecular changes seen primarily in those cells, have also cemented themselves as standard treatments for many cancers.
Over the past several years, immunotherapy therapies that recruit and reinforce the patient’s own immune system to attack tumors, has emerged as what many in the cancer community now call the “fifth pillar” of cancer treatment.

This "fifth pillar" for cancer treatment is the rapidly emerging immunotherapy approach called Adoptive Cell Transfer (ACT): collecting and using patients’ own immune cells to treat their cancer. There are several types of ACT such as Tumor Infiltrating Lymphocytes (TILs), engineered T Cell Receptor (TCRs), Chimeric Antigen Receptor T cell (CAR-T) and Natural Killer cells (NK).
CAR-T (Chimeric Antigen Receptor) for example, shows unprecedented clinical efficacy in Leukemia and Lymphoma, where more than 80% of end stage patients showed remission, and 50% proved to be cancer free 8 years post treatment.
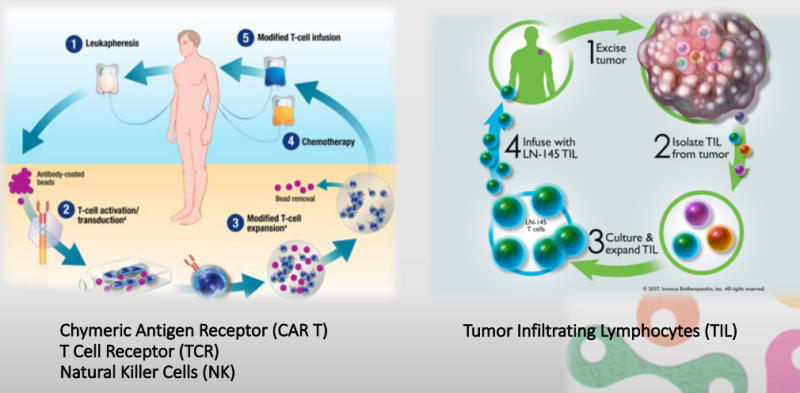
Yet, the super-advanced therapies are not only limited by price (about $500,000 per treatment) and costs - but mostly limited by scale. The therapies are personal and the processing is currently done manually in clean rooms with highly skilled trained and qualified engineers, making the ability to scale up of the technology and expansion of the market very limited.
These new and clinically effective cancer therapies can change the way modern medicine fights cancer but its delivery limited. At this time there is a large scale need for innovative enabling technology, that will dramatically cut down the cost allow for greater availability, ensure quality and availability of this lifesaving immune cell therapy.
The Solution
ADVA biotechnology has developed a noval disruptive technology which enables personalized manufacturing at scale, dramatically reduces cost of production and allows treating patients at the bedside.
ADVA X3 is an innovative, robust and modular cell manufacturing system which enables autologous cell therapy manufacturing, allowing optimization, tight quality assurance, cost reduction and bedside manufacturing.
The device allows automated and closed personalized manufacturing resulting in a reduced cost, reduced need for qualified technicians and enables scaling out the manufacturing to a decentralized model. The integration of the device will revolutionize the manufacturing and increase the availability of the therapy by several folds, enabling evaluability of a real cure for cancer. The simple scaling out model, simple processing and minimal infrastructure needs allows shorter time to market and increases the market size dramatically.
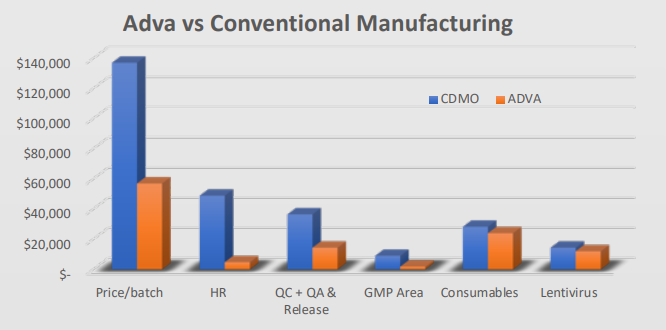
ADVA X3 allows for more than 70% cost reduction, resulting in over $90,000 savings per run, with the major affected cost drivers being the human resource, and GMP area expenditure. As this device is by nature automatic, it has an integrated and validated electronic batch record with automatic monitoring, cutting the costs for QA release and enabling remote quality assurance.
Additional savings can be seen in consumables and probably in the amount of viruses needed for the process. As the system allows optimization of feeding and culture conditions, it is safe to assume that there will be a bigger reduction in materials and virus needed due to optimization.
This cost reduction of over $90,000 basically builds an ROI (Return on Investment) for the device within one to two months of operation.
The technology allows several revenue stream models:The Revenue Model
1. Hardware and software sales/license
2. Hardware and software support and service
3. Disposable
a. Single use elements including chambers, vessels, tubes, sensors and bags
b. In case of partnering with a raw material supplier the packaged raw materials such as media can be provided by the company, adding a revenue stream.
4. Custom development services for specific processes.
Decentralized Manufacturing Model
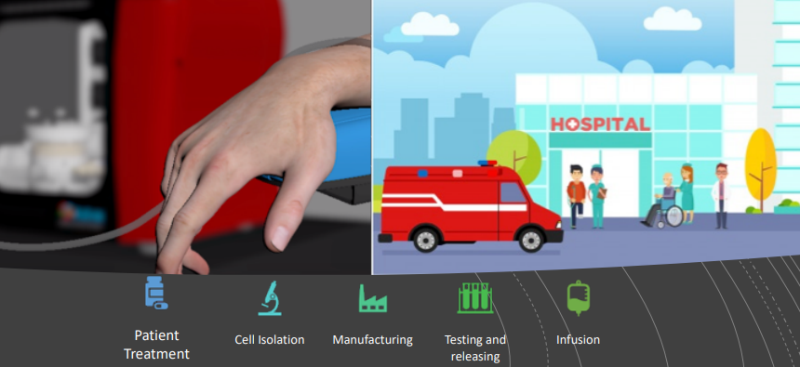
To date, all the manufacturing for cells needed for these treatments is done in a centralized way in a CDMO’s (Contract Development and Manufacturing Organizations) or by the sponsor adding logistic complexity, cost, highly skilled staff, expensive infrastructure and therefore limiting the availability.
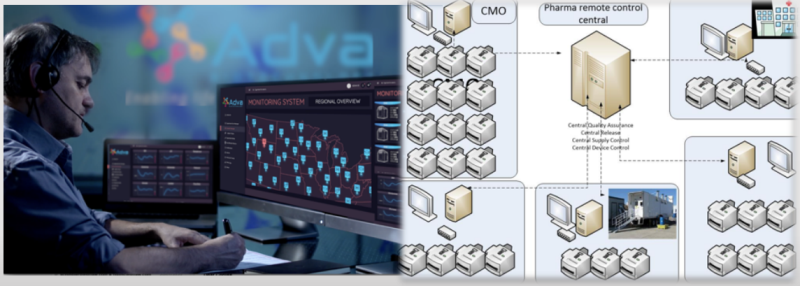
ADVA's technology enables a decentralized manufacturing model where the product is manufactured at the hospitals or near the patients reducing the logistic burden and cost, simplifying the process and ensuring availability to patients. Initially the products will be manufactured using the technology in the central facility, then gradually, it will move to more remote facilities run and managed by the CMO/Sponsor.
Adaptive Cell Therapies are very similar in nature to bone marrow transplants which are done routinely in hospitals. The company believes that in time, along with the clinical and regulatory adaption and new therapies evolving in academic centers and hospitals, the tech will enable new clinical and commercial pathways.
Additionally, the increase in public pressure to allow use of the therapy at lower cost and higher
availability, along with the facts that hospitals are investing money in clean rooms and that these therapies originated in hospitals, the technology will move to application within hospitals, near the patients - thus dramatically increasing the market size and availability.
The EU's ATMP updated guideline (2018) allow manufacturing at Class D of closed system therapies. This change can reduce the cost of infrastructure and operating expense by 70% compared to the class B which is commonly used. These regulatory changes are dramatic and in the upcoming future, the decentralized model will become the main manufacturing model, leading to a larger market for the ADVA device and disposables.
To date, 48% of the CAR T therapies are done in China in a manual form in hospitals allowing
wider access to the therapy. The ADVA X3 system will allow rapid growth and treatment of more
patients in a safe and cost-effective manner.

The Technology
ADVA's technology has been developed over the last 4 years based on model cells (cell line) to optimize the physical parameters of the design. In the past 12 months, real healthy donor CAR T processes using activation beads and antibodies had been tested internally, showing processing results similar and, in some cases, superior to the gold standard, serving as proof of concept.
In addition, joint testing and optimization of the technology with customers that have Autologous immune cell therapies - CAR T, NK, TIL - already started working with ADVA Biotechnology, jointly optimizing the parameters for their process. Currently, a fully integrated beta prototype is being tested and optimized. The company plans to send a few beta V4 prototypes starting September 2020 to chosen beta testers, in order to receive their feedback prior to finalization of the ADVA X3 product and soft launch at Q1/2021.
Timeline and Milestones
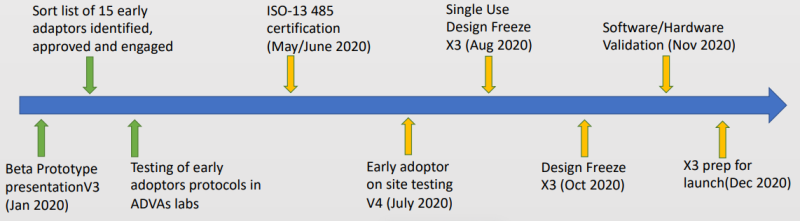
IP and Patents
In August 2016, a provisional patent application was submitted by the company to the USPTO, followed by a worldwide full patent application in Aug 2017. In April 2019 an additional patent application was filed, further strengthening the IP protection.
During Jan 2020 two more applications were finalized and submitted, and in March 2020 an additional application was filed with relevance to the COVID-19 situation, was submitted.
Comparative Analysis
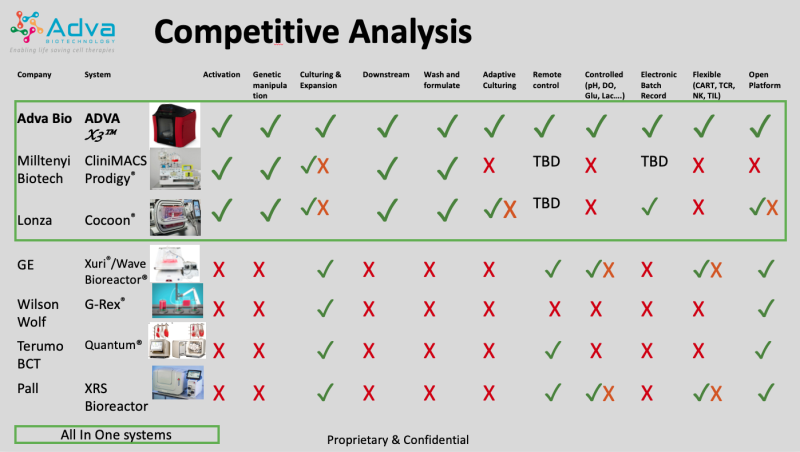
As this table shows, the ADVA X3 is the only system in the field that integrates and controls Temperature, pH, Oxygen, Glucose, lactate, Glutamine, Glutamate, Pressure and Flow rates which are the basis of the automation. These multiple sensors enable artificial intelligence and machine learning to enable optimized culturing.
The vast majority of the technologies in the market are based on scaled down biological manufacturing tools that were not designed for immune cell therapy and are based on old technologies. The meaning of this is that none of the available tools are optimal or supply a full solution.
The two most recent devices which claim to give a wider solution are the “Prodigy” (by Miltenyi Biotech®) which was designed to separate cells and wash post separation. The fact that cells can be cultured in it is a “by product” therefore not optimal nor controlled, and limited in output (1-2 billion cells).
The second device is the “Cocoon®” by Octane Bio and Lonza. It was developed to culture adherent cells and is being modified to fit immune cells but is very limited and complex. It has been in development for over 10 years and has not reached the market, therefore it’s real capabilities, cost or efficiency are unknown. Additionally, the Cocoon® is owned by Lonza which intends to have it as a competitive tool for its CMO, which will limit its availability to the market, since it’s designed for centralized manufacturing.
The ADVA X3 is an enabling revolutionary technology which changes the way cells are being cultured, and unlike competitors, the device has been developed to be a generic device, allowing flexibility and culturing of several cell technologies. Among the types of cells and applications that can be cultured in the system are autologous mesenchymal stem cells, personal gene therapy, exosomes and any embryo bodies or cell clusters. The system can become the next gold standard for cell culturing and can be upscaled to enable allogeneic therapies. The above applications are part of the original patent and will be further developed once the CAR T device reaches the market.
Team
Team
|
A well-known international expert in cell therapy. With over 19 years in the cell therapy industry, Dr Karnieli is the founder of Atvio Biotech, a leading Innovation center for cell & gene therapy that was enquired by a public company. Former VP of Technology & Manufacturing at Pluristem Therapeutics, and the former VP medical device at Goji solutions. Dr Karnieli served as the chair of the process & product development committee of the International Society for Cellular Therapies, expert member in the ISO TC276 Biotechnology standard committee and the former chair of the science and technology committee of the Alliance for Regenerative Medicine.
|
|
Mr. Bercovich has over 17 years of cell therapy development and cGMP experience with extensive expertise in Bioreactor development. Mr. Bercovich was the former manager of Process Engineering at Pluristem Therapeutics and served as the Development Director of Atvio Biotech. Mr. Bercovich’s vast and unique knowledge and experience in cell therapy bioreactor development cGMP cell therapy manufacturing is a critical asset for the company.
|
|
Mrs. Toledo is a certified lawyer and holds an LLB from the Haifa University school of law. Additionally, Mrs. Toledo is a certified and acting mediator. Prior to joining ADVA Biotechnology,
Mrs. Toledo was the Business Development Manager at Atvio Biotech ltd where she build a global BD activity. Prior to Atvio, Ofra was part of the legal and IP department at Pluristem Therapeutics. Ofra brings to the team expertise and experience in BD and marketing in cell therapy.
|
|
Mr. Lindner was the maintenance manager at ATVIO Biotech where he was part of the innovative bioreactor development team. Furthermore, Moshik has over 6 years of experience in maintenance of cGMP production facilities at Pluristem Therapeutics and deep knowledge and expertise in Bioreactors.
|
|
Dr Paldus is the chair of Skehmet Ventures and general partner of Skymoon Ventures. Former Founder, chairman and CEO of Finesse Solutions Inc., a leading bioreactor company recently sold to ThermoFisher Scientific. Dr Paldus was the former founder and CTO of Picarro. Dr Paldus earned her PhD in electronic engineering from Stanford University. Dr Paldus has extensive understanding in the field of biotechnology, bioreactors and a strong and relevant business experience.
|
|
Prof Karnieli is a medical professor from the Technicon Institute, well known world expert in Endocrinology and personalized medicine. Chair of several international and Israeli medical societies in Endocrinology, Telemedicine and Personalized medicine. Head of the Galil center for telemedicine and an advisor to many of the large pharma on personalized medicine and endocrinology. Over 100 pear reviewed papers and books and several on cell & gene therapy.
|
|
Dr Elazary is an experienced entrepreneur in the medical device and energy arena with over 30 approved patents. Dr Elazary is the founder of several companies in the field of medical devices and an advisor and board member over 10 medical device companies. Dr Elazary’s experience and creative thinking provides the company guidance and expertise. relevant business experience.
|
|
Mr. Slonim is the founder of Meitar Engineering with over 25 years of experience in medical and biological device development. Mr. Slonim was a Founder and Co-Inventor in several medical device companies with cutting edge technologies. Tal holds a B.Sc. cum laude from the faculty of mechanical engineering in BGU and MBA from BIU.
|







