NanoVation-GS
Preventing exacerbation of chronic lung disease
109% of funding target


Highlights
Highlights
Like ECG, but for the Lungs: Breakthrough Technology for Monitoring and Managing Chronic Lung Diseases
NanoVation-GS has developed groundbreaking technology for remote monitoring of chronic lung diseases, focusing first on obstructive chronic diseases, such as COPD (Chronic Obstructive Pulmonary Disease). Similarly to how an ECG test enables a comprehensive in-depth examination of the heart function, SenseGuard™ - the system, offered by NanoVation-GS, enables a comprehensive in-depth assessment of breathing and respiratory function.
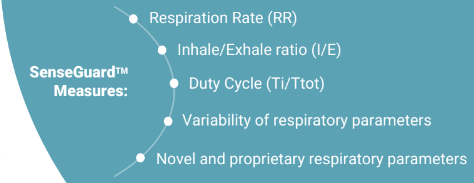
The solution is based on advanced nano-sensor technology, which is particularly sensitive to breathing. Unlike other currently existing solutions, SenseGuard™ enables the measurement of multiple critical and highly relevant to COPD respiratory parameters from the patient's normal breathing without causing discomfort or requiring significant effort from the patient's side. These parameters include well-known as well as novel respiratory biomarkers which were identified and developed using the company's unique nano-sensor technology, and which represent part of its attractive IP. Collected data is transmitted to the cloud where it is transferred into reports, allowing doctors to receive alerts, review and analyze this data from any location, and in parallel is accumulated in a "Big Data" database.
Patented Medical Technology, Developed at the Technion by a Team of World-Class Scientists and Engineers
The company's product is based on a patented technology that was discovered at the Technion and exclusively licensed to NanoVation-GS. The company was founded at the Technion by a team of nanomaterial experts, which, among others, included Dr. Gregory Shuster - NanoVation-GS's founder and CEO, senior researcher in nanomaterials and material engineering, and Prof. Hossam Haick - NanoVation-GS's founder and CSO, leading researcher and expert in nanotechnology. Since its establishment, NanoVation-GS submitted two additional patent applications (pending) which were developed and are owned by the company.

Grants and Investment by the European Innovation Council (EIC) and Israel Innovation Authority
NanoVation-GS has received a prestigious development grant from the EIC, that supports only high-impact start-ups in scaling up their game-changing technologies. As a next step, the EIC Fund has also invested in NanoVation-GS to accelerate the company's development and growth further, making NanoVation-GS the first Israeli company to receive both grant and equity funding - an honor reserved for only 2% of all EIC applicants. The investment agreement also includes EIC's commitment to making an additional investment, in accordance with the terms of the agreement. Prior to that, the company also received grants from the Israel Innovation Authority.

Successful Clinical Trials in 8 Leading Medical Centers in Israel and Abroad
The company's product, the SenseGuard™, has been tested in multiple clinical trials, conducted by NanoVation-GS with medical centers in Israel and in Europe: Halle-Saale University Hospital in Germany; Nicosia Lung Center in Cyprus; Galilee Medical Center, Ichilov Medical Center, Barzilai Hospital, Nazareth Hospital, Poriya Hospital and Rambam Hospital in Israel. The earlier trials demonstrated the safety and usability of the system, and also validated that the accuracy of the product in measuring the Respiratory Rate (RR) is as good as the "gold standard". Further clinical trials have demonstrated the system's ability to monitor and analyze changes in a patient's respiratory conditions based on the novel critical lung function biomarkers developed by NanoVation-GS. Moreover, the trials confirm that the ease and simplicity of use significantly contribute to patients' ability and compliance to use the product and to perform frequent measurements.

Investing in Technology that Received CE and ISO Regulatory Approvals
NanoVation-GS has completed the required procedures in order to obtain necessary regulatory approvals, including CE-mark and ISO13485 certifications. The company is working to update its existing CE certification to the new European regulation (MDR) and is planning to obtain FDA approval later on. NanoVation-GS is ready to start marketing activities in Europe to enter the COPD monitoring market, which is its initial target market. Over time, the company will address further territory markets and will explore the product's scalability for additional commercial and clinical applications, such as monitoring of other respiratory diseases as well as other sectors such as cardiology, pulmonary rehabilitation, sleep research, breathing monitoring for sports, breathing exercises and more.
Improving Quality of Life for Hundreds of Millions of Patients, while Allowing Tens of Billions Financial Savings for Healthcare Providers
Hundreds of millions of people worldwide are currently suffering from respiratory diseases, such as COPD, asthma, pneumonia, cystic fibrosis, COVID-19, and others, that negatively influence patients' quality of life and cause increased, and constantly growing, medical and financial burden on the health care systems. NanoVation-GS's lung function monitoring solution, SenseGuard™, will allow reducing this burden by early detection of pending respiratory exacerbations, which are the main reason for frequent and highly expensive hospital admissions and for the further health status worsening of COPD patients. SenseGuard™ will also help to shorten the duration of COPD patients’ hospitalizations, which cost tens of billions of dollars annually for the healthcare systems in just Europe and the US alone.
Pitch
Pitch
Chronic Obstructive Pulmonary Disease (COPD) is a group of chronic lung diseases, that cause difficulties in breathing. COPD is considered one of the most common chronic medical conditions in the world and the fourth leading cause of death. COPD is periodically punctuated by exacerbations, characterized by acute worsening of symptoms. Despite the prevalence of COPD (as well as other chronic respiratory diseases such as asthma, cystic fibrosis, etc.) and the numerous resources directed to COPD treatment, the market is still missing an effective solution that would help to reduce the high medical and financial burdens associated with COPD due to a large number of emergency department visits, prolonged and frequent hospital admissions and high readmission rates of COPD patients.
Currently, existing technologies and solutions are limited in their abilities and performance and do not allow effective and timely detection of an exacerbation at its early stage. If not treated early, pending exacerbations evolve into an acute state, when the patient's condition becomes even worse and hospitalization is required, which aggravates his suffering and degrades his quality of life in addition to the significant financial burden that healthcare providers and health insurance companies bear (this financial burden is estimated at tens of billions of dollars annually in Europe and the United States alone).
The already high rates of COPD patients' hospitalizations and their long hospitalization durations are supplemented by the extremely high readmission rates: 22% of patients discharged from the hospital after COPD exacerbation will return to the hospital with an additional exacerbation within less than 1 month, and 50% of them within 6 months. Unplanned readmissions that happen within 30 days from discharge are considered as “early readmissions” and in most cases are not reimbursed/covered by the health insurance and instead are paid for by the hospitals themselves. The annual amounts that hospitals lose due to these early readmissions of COPD patients reach billions of dollars in the US alone.
The Solution
SenseGuard™ - a novel wearable medical device, developed by NanoVation-GS to meet current market needs, is based on innovative nano-sensor technology, and is intended for periodic spot-check monitoring of lung function parameters and of respiratory conditions.
SenseGuard™ is designed to help objectively assess patient's respiratory condition, track trends and changes in the condition, identify pending exacerbations, evaluate the effectiveness of ongoing treatment and make timely decisions in order to reduce hospitalizations and early readmissions, optimize the length of stay of patients with chronic respiratory conditions, enhance the value and quality of care, improve the safety, life quality and life expectancy of patients with respiratory diseases, and reduce the medical and financial burdens on health systems.
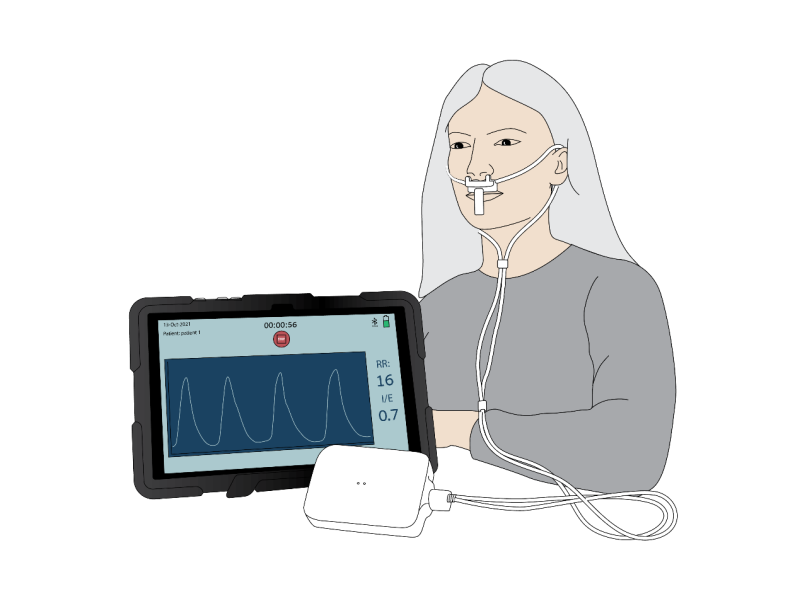
SenseGuard™ has no existing analogs on the market, as due to its unique propriety nano-based respiratory sensors, it seamlessly and without discomfort monitors patient’s normal (tidal) breathing, extracting and analyzing multiple well-known respiratory parameters, and also novel respiratory parameters, that are particularly relevant to COPD, and which were developed by NanoVation-GS and are part of its exclusive intellectual property.
SenseGuard™ is a wireless device, which is easily worn and operated by the patient for comfortable, intuitive, and simple measurements, whether at home or in healthcare settings (clinics, hospitals, nursing homes, etc.).
The measurement process is non-invasive, does not require any effort on the part of the patient, and is adapted for self-measurements carried out by elderly patients without any special support or professional supervision.
Measurement results are uploaded to the cloud platform, to enable remote data review and generation of alerts on changes in patients' conditions.
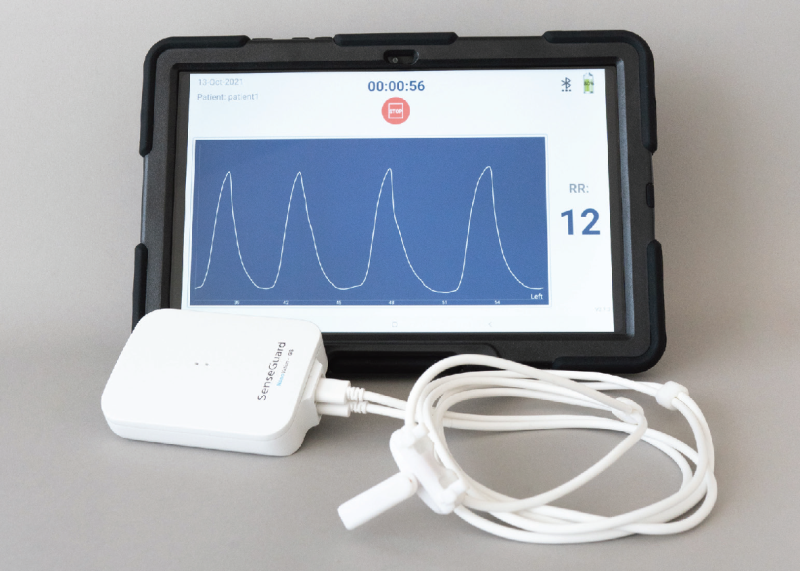
Technology
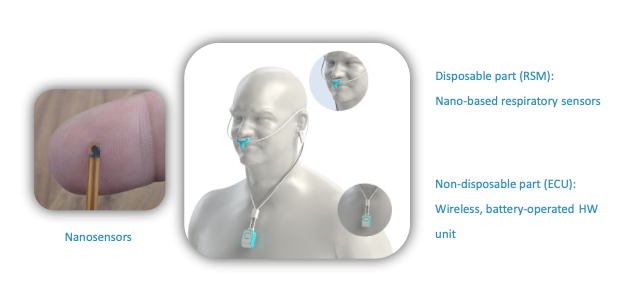
NanoVation-GS's core technology is the nano-based respiratory sensors that were developed and patented at the Technion and exclusively licensed to the company. These unique, highly sensitive sensors are designed for respiratory monitoring purposes, and allow measurement and quantification of various respiratory and lung function parameters among which are multiple well-known respiratory parameters, and also several novel and critical lung function parameters, developed by the company.
The SenseGuard™ device consists of nano-based sensors and hardware components integrated into a compact, lightweight, and unobtrusive wearable design that allows performing routine short and long measurements in a simple, fast and non-invasive way.
The functional design of the device, as well as the new respiratory parameters developed by the company, are protected by patents filed and owned by NanoVation-GS (pending).

SenseGuard™ transmits the collected data automatically and wirelessly to a mobile device and from there the data is uploaded to the cloud (and to the Electronic Medical Records once integration with the healthcare provider is in place) for processing. The cloud platform, built in accordance with HIPAA and GDPR requirements, includes various alert and processing algorithms.
The clinical information is processed and transferred into detailed reports using proprietary algorithms uniquely owned by the company. The reports are accessible to doctors from anywhere and enable them to see the information on patients’ conditions, identify trends, and set threshold alerts on specific measurement results or on-trend changes.
Team
Team
|
Prof. Haick is one of NanoVation-GS‘s co-founders and CSO. He is an expert in the field of nanotechnology and non-invasive disease diagnosis. His studies include the research and development of nano-array devices for screening, diagnosis, and monitoring of disease, nanomaterial-based chemical (flexible) sensors, electronic skin, breath analysis, volatile biomarkers, and cell-to-cell communication. His studies have generated more than 220 publications in the last ten years in top-level journals in the field of nanotechnology, advanced/applied materials/chemistry, and medicine, and more than ten book chapters. Since he joined the Technion, Prof. Haick‘s articles received more than 14000 citations, 37 of which with >100 citations. His H-Index ~67 (Google Scholar).
The technologies developed by Prof. Haick and his team have led to the production of more than 40 patents and patent applications – many of which have been licensed to international companies and ground for startup companies.
Prof. Haick has received more than 70 prizes and recognitions, including the Knight of the Order of Academic Palms (conferred by the French Government), the Humboldt Senior Research Award, the EU’s Electronic Components & Systems (ECS) Innovation Award, the Changjiang Award, the “Michael Bruno” award for truly exceptional scientists, etc. He was also included in more than 40 important ranking lists, such as the of the world’s 35 leading young scientists by MIT Magazine (2008), top-100 innovators in the world (2015-2018) by various international organizations, etc.
Hossam Haick is a Full Professor in the Technion and the Dean of Undergraduate Studies. He earned his doctorate from the Technion in 2002. After graduation he did two 2-years long postdoctoral fellowships - first at the Weizmann Institute of Science, then at the California Institute of Technology (Caltech).
|
|
Prof. Dr. med. Felix Herth is Medical Advisor of NanoVation-GS since 2019.
Prof. Dr. med. Felix Herth is a renowned specialist, researcher, and head of one of the leading clinics in the field of the development of new invasive endoscopic methods. His research group has developed many new diagnostic methods, among which are endobronchial sonography, navigated bronchoscopy, analysis of epithelial mucus of the lungs, intrabronchial use of therapeutic drugs, etc. He is the author of numerous publications.
Prof. Dr. med. Felix Herth is CEO and Chief Medical Officer of the Thoraxklinik Heidelberg and Chief Physician of the department for Internal Medicine – Pneumology of the Thoraxklinik Heidelberg, which is part of the local University hospital. His research interest focuses on interventional pneumology and the treatment of COPD.
|
|
Nadav is the Technical Leader of NanoVation-GS and he successfully led the company through ISO13485 certification, product and technology development, production, and regulatory approval processes since 2016. Nadav holds both a B.Sc. and an M.Sc. degree in Chemical Engineering from the Technion, specializing in Nanotechnology, and has deep expertise in research, process development, quality, and regulation in the field of medical devices. Nadav has published several peer-reviewed papers in high-ranked journals on his work with nanotechnology-based sensors. Prior to joining NanoVation-GS, he worked in the semiconductors industry.
|
|
Prof. Voigt joined NanoVation-GS in 2019 after serving in different positions, including Head of Clinical Strategy and Chief Medical Officer at Siemens Healthineers. He is Head of Innovation in Oncology at Steinbeis University Berlin, an experienced Clinical Expert and Medical Advisor, and an internationally renowned and well-published expert in Medical Technologies.
Prof. Voigt has vast experience in cross-business line clinical R&D and marketing support: during his career, he was driving clinical studies in the field of outcome research with medical technology, clinical product evaluations, clinical sales support, KOL interaction, and networking, medical society relationships development and support.
Prof. Voigt took an active part in the development of a clinical strategy for Siemens Healthineers and the execution of prioritized projects of the clinical strategy, which turned him into a Key Expert in the development of clinical strategies, and identification of clinical trends & unmet medical needs as fields for new business opportunities.
Prof Voigt has a long-term experience in hospital strategy & operational process planning, hospital process and clinical workflow development, and continuous quality improvement and optimization.
Prof. Voigt also contributes his vast knowledge in conducting clinical evaluations as part of the regulatory approval process.
Prof. Voigt is a board-certified specialist for Internal Medicine and a Professor for Innovation with more than 15 years of clinical experience as a senior consultant at a University Hospital.
|
|
Dr. Yuval Avni is NanoVation-GS‘s Business Development Advisor. Dr. Avni has over 15 years of experience on both sides of the table in high-tech and innovation as an entrepreneur, executive, and investor. He is Managing General Partner at Crescendo Venture Partners and Partner and Co-founder at Swanlaab Venture Factory (Spain), a Venture Capital Fund investing in innovative Spanish companies.
In the past, Dr. Avni served as CEO at Beta-O2 Technologies, Ltd. He also was a partner at Giza VC, a leading early-stage Israeli venture capital firm with hundreds of millions of dollars under management, with a focus on the life science and clean tech sectors. Dr. Avni was involved in the entire investment process – sourcing, screening, and due diligence. He led or co-led investments in many technology companies, supporting them from investment through the exit. Dr. Avni also led Giza’s international business development group and was part of the founding team of the firm’s sponsored VC funds worldwide.
In addition to vast experience as a venture capitalist, Dr. Avni is also a trained physician, with clinical experience in general and vascular surgery. He has conducted clinical research in the areas of human genetics, general surgery, and vascular surgery.
Dr. Avni is also a visiting lecturer on entrepreneurship and fundraising at the Technion – Israel Institute of Technology – and Tel Aviv University.
Dr. Avni received his medical training at the Technion and holds a Bachelor’s degree (with honors) from the Technion in medical science.
|
|
Masha joined the team in 2019 to take over the business development and marketing activities of NanoVation-GS and to continue leading SenseGuard™ market entry and commercialization. Masha is a sales and marketing executive with more than 20 years of managing experience as senior BD & Sales Director at international companies such as Honeywell and Ingersoll Rand. In the last few years before joining the NanoVation-GS team, Masha served as external business development and sales consultant for start-up companies in Israel and Russia. She holds BA in Management from TA University.
|






